当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
General Installation of (4H)-Imidazolone cis-Amide Bioisosteres Along the Peptide Backbone
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2024-04-17 , DOI: 10.1021/jacs.3c13825 Brendan J. Wall 1 , Krishna K. Sharma 1 , Emily A. O’Brien 1 , Aaron Donovan 1 , Brett VanVeller 1
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2024-04-17 , DOI: 10.1021/jacs.3c13825 Brendan J. Wall 1 , Krishna K. Sharma 1 , Emily A. O’Brien 1 , Aaron Donovan 1 , Brett VanVeller 1
Affiliation
|
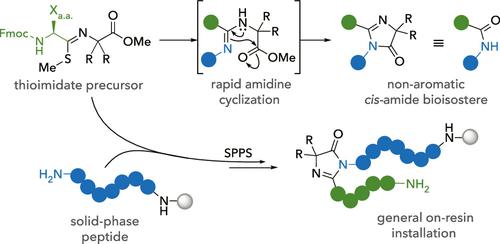
Imidazolones represent an important class of heterocycles present in a wide range of pharmaceuticals, metabolites, and bioactive natural products and serve as the active chromophore in green fluorescent protein. Recently, imidazolones have received attention for their ability to act as a nonaromatic amide bond bioisotere which improves pharmacological properties. Herein, we present a tandem amidine installation and cyclization with an adjacent ester to yield (4H)-imidazolone products. Using amino acid building blocks, we can access the first examples of α-chiral imidazolones that have been previously inaccessible. Additionally, our method is amenable to on-resin installation which can be seamlessly integrated into existing solid-phase peptide synthesis protocols. Finally, we show that peptide imidazolones are potent cis-amide bond surrogates that preorganize linear peptides for head-to-tail macrocyclization. This work represents the first general approach to the backbone and side-chain insertion of imidazolone bioisosteres at various positions in linear and cyclic peptides.
中文翻译:

(4H)-咪唑酮顺酰胺生物等排体沿着肽主链的一般安装
咪唑啉酮是一类重要的杂环化合物,存在于多种药物、代谢物和生物活性天然产物中,并充当绿色荧光蛋白中的活性发色团。最近,咪唑酮因其作为非芳香族酰胺键生物电子等排体的能力而受到关注,从而改善药理学特性。在此,我们提出了串联脒装置并与相邻酯环化以产生 (4 H )-咪唑啉酮产物。使用氨基酸构建模块,我们可以获得以前无法获得的 α-手性咪唑啉酮的第一个例子。此外,我们的方法适合树脂安装,可以无缝集成到现有的固相肽合成方案中。最后,我们证明肽咪唑啉酮是有效的顺式酰胺键替代物,可以预组织线性肽以进行头尾大环化。这项工作代表了在线性和环状肽的不同位置上咪唑啉酮生物电子等排体的主链和侧链插入的第一个通用方法。
更新日期:2024-04-17
中文翻译:

(4H)-咪唑酮顺酰胺生物等排体沿着肽主链的一般安装
咪唑啉酮是一类重要的杂环化合物,存在于多种药物、代谢物和生物活性天然产物中,并充当绿色荧光蛋白中的活性发色团。最近,咪唑酮因其作为非芳香族酰胺键生物电子等排体的能力而受到关注,从而改善药理学特性。在此,我们提出了串联脒装置并与相邻酯环化以产生 (4 H )-咪唑啉酮产物。使用氨基酸构建模块,我们可以获得以前无法获得的 α-手性咪唑啉酮的第一个例子。此外,我们的方法适合树脂安装,可以无缝集成到现有的固相肽合成方案中。最后,我们证明肽咪唑啉酮是有效的顺式酰胺键替代物,可以预组织线性肽以进行头尾大环化。这项工作代表了在线性和环状肽的不同位置上咪唑啉酮生物电子等排体的主链和侧链插入的第一个通用方法。



























 京公网安备 11010802027423号
京公网安备 11010802027423号