当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Rapid Enrichment of a Native Multipass Transmembrane Protein via Cell Membrane Electrophoresis through Buffer pH and Ionic Strength Adjustment
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2024-04-17 , DOI: 10.1021/jacs.3c13579 Tzu-Tzu Liu, Sin-Han Huang, Ling Chao
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2024-04-17 , DOI: 10.1021/jacs.3c13579 Tzu-Tzu Liu, Sin-Han Huang, Ling Chao
|
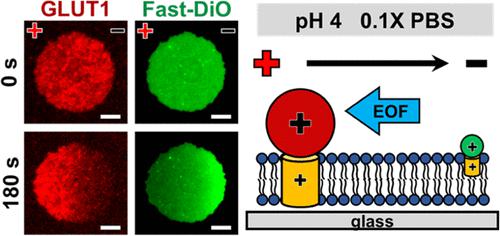
Supported membrane electrophoresis is a promising technique for collecting membrane proteins in native bilayer environments. However, the slow mobility of typical transmembrane proteins has impeded the technique’s advancement. Here, we successfully applied cell membrane electrophoresis to rapidly enrich a 12-transmembrane helix protein, glucose transporter 1 with antibodies (GLUT1 complex), by tuning the buffer pH and ionic strength. The identified conditions allowed the separation of the GLUT1 complex and a lipid probe, Fast-DiO, within a native-like environment in a few minutes. A force model was developed to account for distinct electric and drag forces acting on the transmembrane and aqueous-exposed portion of a transmembrane protein as well as the electroosmotic force. This model not only elucidates the impact of size and charge properties of transmembrane proteins but also highlights the influence of pH and ionic strength on the driving forces and, consequently, electrophoretic mobility. Model predictions align well with experimentally measured electrophoretic mobilities of the GLUT1 complex and Fast-DiO at various pH and ionic strengths as well as with several lipid probes, lipid-anchored proteins, and reconstituted membrane proteins from previous studies. Force analyses revealed the substantial membrane drag of the GLUT1 complex, significantly slowing down electrophoretic mobility. Besides, the counterbalance of similar magnitudes of electroosmotic and electric forces results in a small net driving force and, consequently, reduced mobility under typical neutral pH conditions. Our results further highlight how the size and charge properties of transmembrane proteins influence the suitable range of operating conditions for effective movement, providing potential applications for concentrating and isolating membrane proteins within this platform.
中文翻译:

通过调整缓冲液 pH 和离子强度,通过细胞膜电泳快速富集天然多次跨膜蛋白
支持膜电泳是一种在天然双层环境中收集膜蛋白的有前途的技术。然而,典型跨膜蛋白的缓慢移动性阻碍了该技术的进步。在这里,我们成功地应用细胞膜电泳,通过调节缓冲液 pH 值和离子强度,用抗体(GLUT1 复合物)快速富集 12 次跨膜螺旋蛋白、葡萄糖转运蛋白 1。确定的条件允许在几分钟内在类似天然的环境中分离 GLUT1 复合物和脂质探针 Fast-DiO。开发了一个力模型来解释作用于跨膜蛋白的跨膜和水暴露部分的不同电力和阻力以及电渗力。该模型不仅阐明了跨膜蛋白的大小和电荷特性的影响,而且还强调了 pH 值和离子强度对驱动力以及电泳迁移率的影响。模型预测与实验测量的 GLUT1 复合物和 Fast-DiO 在各种 pH 和离子强度下的电泳迁移率以及之前研究中的几种脂质探针、脂质锚定蛋白和重构膜蛋白吻合良好。力分析揭示了 GLUT1 复合物的大量膜阻力,显着减慢了电泳迁移率。此外,相似大小的电渗力和电力的平衡导致净驱动力较小,因此在典型的中性 pH 条件下流动性降低。我们的结果进一步强调了跨膜蛋白的大小和电荷特性如何影响有效运动的合适操作条件范围,为该平台内浓缩和分离膜蛋白提供了潜在的应用。
更新日期:2024-04-17
中文翻译:

通过调整缓冲液 pH 和离子强度,通过细胞膜电泳快速富集天然多次跨膜蛋白
支持膜电泳是一种在天然双层环境中收集膜蛋白的有前途的技术。然而,典型跨膜蛋白的缓慢移动性阻碍了该技术的进步。在这里,我们成功地应用细胞膜电泳,通过调节缓冲液 pH 值和离子强度,用抗体(GLUT1 复合物)快速富集 12 次跨膜螺旋蛋白、葡萄糖转运蛋白 1。确定的条件允许在几分钟内在类似天然的环境中分离 GLUT1 复合物和脂质探针 Fast-DiO。开发了一个力模型来解释作用于跨膜蛋白的跨膜和水暴露部分的不同电力和阻力以及电渗力。该模型不仅阐明了跨膜蛋白的大小和电荷特性的影响,而且还强调了 pH 值和离子强度对驱动力以及电泳迁移率的影响。模型预测与实验测量的 GLUT1 复合物和 Fast-DiO 在各种 pH 和离子强度下的电泳迁移率以及之前研究中的几种脂质探针、脂质锚定蛋白和重构膜蛋白吻合良好。力分析揭示了 GLUT1 复合物的大量膜阻力,显着减慢了电泳迁移率。此外,相似大小的电渗力和电力的平衡导致净驱动力较小,因此在典型的中性 pH 条件下流动性降低。我们的结果进一步强调了跨膜蛋白的大小和电荷特性如何影响有效运动的合适操作条件范围,为该平台内浓缩和分离膜蛋白提供了潜在的应用。



























 京公网安备 11010802027423号
京公网安备 11010802027423号