当前位置:
X-MOL 学术
›
Mater. Today Phys.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Structural evolution of the conformational ensembles in peptide fibrillar aggregates
Materials Today Physics ( IF 11.5 ) Pub Date : 2024-04-15 , DOI: 10.1016/j.mtphys.2024.101437 Zhongyi Jian , Ruonan Wang , Shanshan Mo , Zhun Deng , Shuyuan Li , Zhenyan Li , Mingzhan Wang , Yanlian Yang , Chen Wang , Wenbo Zhang , Lanlan Yu , Chenxuan Wang
Materials Today Physics ( IF 11.5 ) Pub Date : 2024-04-15 , DOI: 10.1016/j.mtphys.2024.101437 Zhongyi Jian , Ruonan Wang , Shanshan Mo , Zhun Deng , Shuyuan Li , Zhenyan Li , Mingzhan Wang , Yanlian Yang , Chen Wang , Wenbo Zhang , Lanlan Yu , Chenxuan Wang
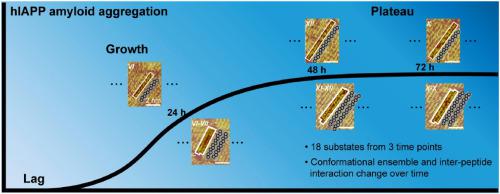
|
Exploring the folding structures and intermolecular recognitions of proteins in their assemblies is essential for revealing the mechanisms of amyloid fibrillar aggregation. However, the molecular-level description of protein structural changes with aggregation progression is challenging due to the heterogeneity of assembled structures. Here we report the use of scanning tunneling microscopy (STM), which is competent in analyzing the highly heterogeneous structures with submolecular resolution, to probe the structures of human islet amyloid polypeptide (hIAPP) assemblies at the different time points of hIAPP fibrillation. The conformational substate ensembles of the β-strands formed by hIAPP as well as the interpeptide interactions are found to change with time in the growth and plateau phases. Detailed analyses of the distribution probability of interpeptide interactions at the different time points manifest that the enthalpic contribution to the structural conversions of β-sheet fibrillation becomes more significant. Our single-molecular level studies highlight the dynamic nature of protein structures within a β-sheet-assembled fibril.
中文翻译:

肽纤维聚集体中构象整体的结构演化
探索蛋白质组装中的折叠结构和分子间识别对于揭示淀粉样蛋白原纤维聚集的机制至关重要。然而,由于组装结构的异质性,蛋白质结构随聚集过程变化的分子水平描述具有挑战性。在这里,我们报告使用扫描隧道显微镜(STM)来探测人胰岛淀粉样多肽(hIAPP)在hIAPP纤维颤动的不同时间点的结构,该显微镜能够以亚分子分辨率分析高度异质的结构。发现 hIAPP 形成的 β 链的构象亚状态整体以及肽间相互作用随着生长阶段和平台期的时间而变化。对不同时间点肽间相互作用分布概率的详细分析表明,焓对β-片层纤维化结构转换的贡献变得更加显着。我们的单分子水平研究强调了 β-折叠组装原纤维内蛋白质结构的动态性质。
更新日期:2024-04-15
中文翻译:

肽纤维聚集体中构象整体的结构演化
探索蛋白质组装中的折叠结构和分子间识别对于揭示淀粉样蛋白原纤维聚集的机制至关重要。然而,由于组装结构的异质性,蛋白质结构随聚集过程变化的分子水平描述具有挑战性。在这里,我们报告使用扫描隧道显微镜(STM)来探测人胰岛淀粉样多肽(hIAPP)在hIAPP纤维颤动的不同时间点的结构,该显微镜能够以亚分子分辨率分析高度异质的结构。发现 hIAPP 形成的 β 链的构象亚状态整体以及肽间相互作用随着生长阶段和平台期的时间而变化。对不同时间点肽间相互作用分布概率的详细分析表明,焓对β-片层纤维化结构转换的贡献变得更加显着。我们的单分子水平研究强调了 β-折叠组装原纤维内蛋白质结构的动态性质。



























 京公网安备 11010802027423号
京公网安备 11010802027423号