Statistical Papers ( IF 1.3 ) Pub Date : 2024-04-17 , DOI: 10.1007/s00362-024-01549-x Andrea Ongaro , Sonia Migliorati , Roberto Ascari , Enrico Ripamonti
|
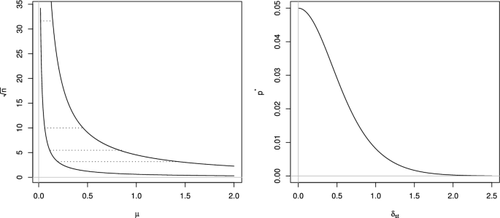
Traditionally, common testing problems are formalized in terms of a precise null hypothesis representing an idealized situation such as absence of a certain “treatment effect”. However, in most applications the real purpose of the analysis is to assess evidence in favor of a practically relevant effect, rather than simply determining its presence/absence. This discrepancy leads to erroneous inferential conclusions, especially in case of moderate or large sample size. In particular, statistical significance, as commonly evaluated on the basis of a precise hypothesis low p value, bears little or no information on practical significance. This paper presents an innovative approach to the problem of testing the practical relevance of effects. This relies upon the proposal of a general method for modifying standard tests by making them suitable to deal with appropriate interval null hypotheses containing all practically irrelevant effect sizes. In addition, when it is difficult to specify exactly which effect sizes are irrelevant we provide the researcher with a benchmark value. Acceptance/rejection can be established purely by deciding on the (ir)relevance of this value. We illustrate our proposal in the context of many important testing setups, and we apply the proposed methods to two case studies in clinical medicine. First, we consider data on the evaluation of systolic blood pressure in a sample of adult participants at risk for nutritional deficit. Second, we focus on a study of the effects of remdesivir on patients hospitalized with COVID-19.
中文翻译:

测试治疗效果的实际相关性
传统上,常见的测试问题是根据代表理想情况(例如不存在某种“治疗效果”)的精确零假设来形式化的。然而,在大多数应用中,分析的真正目的是评估有利于实际相关效果的证据,而不是简单地确定其存在/不存在。这种差异会导致错误的推断结论,特别是在样本量中等或较大的情况下。特别是,统计显着性(通常根据精确假设低p值进行评估)几乎没有或没有实际显着性信息。本文提出了一种创新方法来解决效果的实际相关性测试问题。这依赖于修改标准检验的通用方法的提议,使标准检验适合处理包含所有实际上不相关效应大小的适当区间零假设。此外,当难以准确指定哪些效应大小不相关时,我们为研究人员提供基准值。可以纯粹通过决定该值的(或)相关性来确定接受/拒绝。我们在许多重要的测试设置的背景下阐述了我们的建议,并将所提出的方法应用于临床医学的两个案例研究。首先,我们考虑有营养缺乏风险的成年参与者样本的收缩压评估数据。其次,我们重点研究瑞德西韦对住院的 COVID-19 患者的影响。



























 京公网安备 11010802027423号
京公网安备 11010802027423号