Chem Catalysis Pub Date : 2024-03-08 , DOI: 10.1016/j.checat.2024.100946 Qibin Zhu , Xinyu Tian , Gang He
|
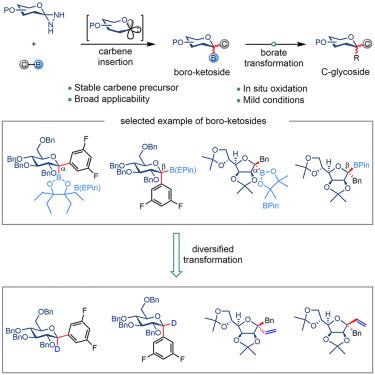
C-glycosides are widely present in natural products and artificially designed therapeutic agents. The synthesis of C-glycosides has attracted considerable attention, and strategies based on the transformation of oxocarbenium ions, glycosyl anions, glycosyl radicals, or transition metal complexes have been extensively studied. Glycosylation based on the chemistry of glycosylidene carbenes, by contrast, is far less explored, and the generation of glycosylidene carbenes is a long-standing challenge. Herein, we report a practical method for the generation of glycosylidene carbenes by copper-catalyzed oxidation of bench-stable glycosylidene diaziridines with oxygen at room temperature. Capturing the glycosylidene carbenes with boronic esters afforded boro-ketosides, which were conveniently converted to structurally diversified C-glycosides via vinylation, deuteration, and oxidation reactions. This procedure offers a powerful platform for exploring the chemistry of glycosylidene carbenes and developing innovative glycosylation strategies.
中文翻译:

糖亚基-卡宾介导的硼酸酯同系化用于合成硼酮苷
C-糖苷广泛存在于天然产物和人工设计的治疗剂中。C-糖苷的合成引起了人们的广泛关注,基于氧碳鎓离子、糖基阴离子、糖基自由基或过渡金属配合物的转化策略已被广泛研究。相比之下,基于糖基卡宾化学的糖基化却很少被探索,并且糖基卡宾的生成是一个长期存在的挑战。在此,我们报告了一种在室温下用氧气对稳定的糖基二氮丙啶进行铜催化氧化生成糖基卡宾的实用方法。用硼酸酯捕获糖基卡宾得到硼酮苷,通过乙烯基化、氘化和氧化反应可以方便地转化为结构多样化的C-糖苷。该过程为探索糖基卡宾的化学性质和开发创新的糖基化策略提供了一个强大的平台。



























 京公网安备 11010802027423号
京公网安备 11010802027423号