当前位置:
X-MOL 学术
›
J. Immunol. Methods
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Development of a defibrinated human blood hemolysis assay for rapid testing of hemolytic activity compared to computational prediction
Journal of Immunological Methods ( IF 2.2 ) Pub Date : 2024-04-09 , DOI: 10.1016/j.jim.2024.113670 Ashley M. Carpenter , Monique L. van Hoek
Journal of Immunological Methods ( IF 2.2 ) Pub Date : 2024-04-09 , DOI: 10.1016/j.jim.2024.113670 Ashley M. Carpenter , Monique L. van Hoek
|
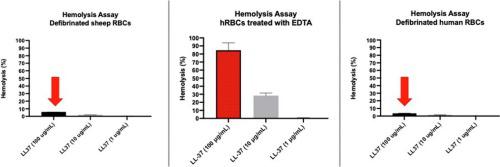
Cytotoxicity studies determining hemolytic properties of antimicrobial peptides or other drugs are an important step in the development of novel therapeutics for clinical use. Hemolysis is an affordable, accessible, and rapid method for initial assessment of cellular toxicity for all drugs under development. However, variability in species of red blood cells and protocols used may result in significant differences in results. AMPs generally possess higher selectivity for bacterial cells but can have toxicity against host cells at high concentrations. Knowing the hemolytic activity of the peptides we are developing contributes to our understanding of their potential toxicity. Computational approaches for predicting hemolytic activity of AMPs exist and were tested head-to-head with our experimental results. Starting with an observation of high hemolytic activity of LL-37 peptide against human red blood cells that were collected in EDTA, we explored alternative approaches to develop a more robust, accurate and simple hemolysis assay using defibrinated human blood. We found significant differences between the sensitivity of defibrinated red blood cells and EDTA treated red blood cells. Accurately determining the hemolytic activity using human red blood cells will allow for a more robust calculation of the therapeutic index of our potential antimicrobial compounds, a critical measure in their pre-clinical development. We introduce a standardized, more accurate protocol for assessing hemolytic activity using defibrinated human red blood cells. This approach, facilitated by the increased commercial availability of de-identified human blood and defibrination methods, offers a robust tool for evaluating toxicity of emerging drug compounds, especially AMPs.
中文翻译:

开发去纤维人血溶血测定法,用于与计算预测相比快速测试溶血活性
确定抗菌肽或其他药物的溶血特性的细胞毒性研究是开发临床使用的新型疗法的重要一步。溶血是一种经济实惠、易于使用且快速的方法,用于对所有正在开发的药物的细胞毒性进行初步评估。然而,红细胞种类和使用的方案的差异可能会导致结果的显着差异。 AMP 通常对细菌细胞具有较高的选择性,但在高浓度下可能对宿主细胞具有毒性。了解我们正在开发的肽的溶血活性有助于我们了解其潜在毒性。预测 AMP 溶血活性的计算方法是存在的,并与我们的实验结果进行了直接测试。从观察 LL-37 肽对 EDTA 中收集的人红细胞的高溶血活性开始,我们探索了替代方法,以使用去纤维的人血开发更稳健、准确和简单的溶血测定。我们发现去纤维红细胞和 EDTA 处理的红细胞的敏感性之间存在显着差异。使用人类红细胞准确测定溶血活性将有助于更可靠地计算我们潜在抗菌化合物的治疗指数,这是其临床前开发的关键措施。我们引入了一种标准化、更准确的方案,用于使用去纤维的人红细胞评估溶血活性。这种方法在去识别的人类血液和去纤维方法的商业可用性增加的推动下,为评估新兴药物化合物(尤其是 AMP)的毒性提供了强大的工具。
更新日期:2024-04-09
中文翻译:

开发去纤维人血溶血测定法,用于与计算预测相比快速测试溶血活性
确定抗菌肽或其他药物的溶血特性的细胞毒性研究是开发临床使用的新型疗法的重要一步。溶血是一种经济实惠、易于使用且快速的方法,用于对所有正在开发的药物的细胞毒性进行初步评估。然而,红细胞种类和使用的方案的差异可能会导致结果的显着差异。 AMP 通常对细菌细胞具有较高的选择性,但在高浓度下可能对宿主细胞具有毒性。了解我们正在开发的肽的溶血活性有助于我们了解其潜在毒性。预测 AMP 溶血活性的计算方法是存在的,并与我们的实验结果进行了直接测试。从观察 LL-37 肽对 EDTA 中收集的人红细胞的高溶血活性开始,我们探索了替代方法,以使用去纤维的人血开发更稳健、准确和简单的溶血测定。我们发现去纤维红细胞和 EDTA 处理的红细胞的敏感性之间存在显着差异。使用人类红细胞准确测定溶血活性将有助于更可靠地计算我们潜在抗菌化合物的治疗指数,这是其临床前开发的关键措施。我们引入了一种标准化、更准确的方案,用于使用去纤维的人红细胞评估溶血活性。这种方法在去识别的人类血液和去纤维方法的商业可用性增加的推动下,为评估新兴药物化合物(尤其是 AMP)的毒性提供了强大的工具。



























 京公网安备 11010802027423号
京公网安备 11010802027423号