当前位置:
X-MOL 学术
›
J. Environ. Chem. Eng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Base-free synthesis of renewable furan-2,5-dicarboxylic acid (FDCA) over an earth-abundant magnetic catalyst
Journal of Environmental Chemical Engineering ( IF 7.7 ) Pub Date : 2024-04-13 , DOI: 10.1016/j.jece.2024.112763 Surabhi Pandey , Marie Mottoul , Valérie Orsat , Jean-François Morin , Marie-Josée Dumont
Journal of Environmental Chemical Engineering ( IF 7.7 ) Pub Date : 2024-04-13 , DOI: 10.1016/j.jece.2024.112763 Surabhi Pandey , Marie Mottoul , Valérie Orsat , Jean-François Morin , Marie-Josée Dumont
|
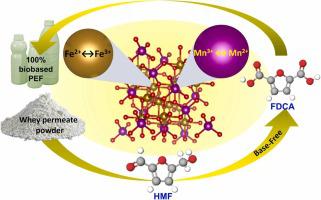
Being a biobased alternative to petroleum-based terephthalic acid (TPA), furan-2,5-dicarboxylic acid (FDCA) requires a low-cost and efficient catalytic system for its commercialization. This study describes FDCA production using whey permeate powder (WPP)-derived 5-(hydroxymethyl)furfural (HMF) in a base-free system over a magnetically recyclable catalyst composed of earth-abundant metals (Mn and Fe). The impact of MnCl·4 HO loading on the iron core was studied at different levels and found optimal at the ratio of 1:1. The in-situ oxidation of HMF was carried out using ozone and -BuOOH as the oxidants in a γ-valerolactone (GVL) -water solvent system. It was observed that the ozone pre-treatment (3 h at room temperature) of HMF increased the FDCA yield from 39.7 % (without ozonation) to 60 % at a reaction condition of 130 °C for 2 h. The concentration profiles for the intermediates formed during HMF to FDCA oxidation i.e., 5-hydroxymethyl-2-furancarboxylic acid (HMFCA), furan-2,5-dicarbaldehyde (DFF), and 5-formyl-2-furancarboxylic acid (FFCA) were analyzed at different reaction temperatures to evaluate the reaction kinetics. The kinetic study revealed that the present solvent-catalytic system favored HMFCA formation over DFF due to the requirement of higher activation energy for the latter. The HMF to FDCA catalytic solvent was tested for WPP, which afforded an overall FDCA yield of 14.6 % at 130 °C in 120 min. In addition, the MnFe consisted of mixed phases of MnO, MnO, MnO, FeO, and FeO and demonstrated variation in oxidation states for Mn (Mn↔Mn) and Fe (Fe↔Fe) species.
中文翻译:

利用地球储量丰富的磁性催化剂无碱合成可再生呋喃-2,5-二甲酸 (FDCA)
作为石油基对苯二甲酸 (TPA) 的生物基替代品,呋喃-2,5-二甲酸 (FDCA) 需要低成本、高效的催化系统才能实现其商业化。本研究描述了在无碱系统中使用乳清渗透粉 (WPP) 衍生的 5-(羟甲基)糠醛 (HMF) 在由地球丰富的金属(锰和铁)组成的磁性可回收催化剂上生产 FDCA。在不同水平上研究了MnCl·4 H2O负载量对铁芯的影响,发现最佳比例为1:1。以臭氧和-BuOOH为氧化剂,在γ-戊内酯(GVL)-水溶剂体系中对HMF进行原位氧化。据观察,HMF 的臭氧预处理(室温下 3 小时)在 130 °C 反应条件下 2 小时将 FDCA 产率从 39.7%(无臭氧化)提高到 60%。 HMF 至 FDCA 氧化过程中形成的中间体的浓度分布,即 5-羟甲基-2-呋喃甲酸 (HMFCA)、呋喃-2,5-二甲醛 (DFF) 和 5-甲酰基-2-呋喃甲酸 (FFCA)在不同反应温度下进行分析以评估反应动力学。动力学研究表明,由于后者需要更高的活化能,因此目前的溶剂催化系统比 DFF 更有利于 HMFCA 的形成。对 WPP 测试了 HMF 至 FDCA 催化溶剂,在 130 °C 下 120 分钟内,FDCA 总产率为 14.6%。此外,MnFe 由 MnO、MnO、MnO、FeO 和 FeO 的混合相组成,并且表现出 Mn (Mn↔Mn) 和 Fe (Fe↔Fe) 物质氧化态的变化。
更新日期:2024-04-13
中文翻译:

利用地球储量丰富的磁性催化剂无碱合成可再生呋喃-2,5-二甲酸 (FDCA)
作为石油基对苯二甲酸 (TPA) 的生物基替代品,呋喃-2,5-二甲酸 (FDCA) 需要低成本、高效的催化系统才能实现其商业化。本研究描述了在无碱系统中使用乳清渗透粉 (WPP) 衍生的 5-(羟甲基)糠醛 (HMF) 在由地球丰富的金属(锰和铁)组成的磁性可回收催化剂上生产 FDCA。在不同水平上研究了MnCl·4 H2O负载量对铁芯的影响,发现最佳比例为1:1。以臭氧和-BuOOH为氧化剂,在γ-戊内酯(GVL)-水溶剂体系中对HMF进行原位氧化。据观察,HMF 的臭氧预处理(室温下 3 小时)在 130 °C 反应条件下 2 小时将 FDCA 产率从 39.7%(无臭氧化)提高到 60%。 HMF 至 FDCA 氧化过程中形成的中间体的浓度分布,即 5-羟甲基-2-呋喃甲酸 (HMFCA)、呋喃-2,5-二甲醛 (DFF) 和 5-甲酰基-2-呋喃甲酸 (FFCA)在不同反应温度下进行分析以评估反应动力学。动力学研究表明,由于后者需要更高的活化能,因此目前的溶剂催化系统比 DFF 更有利于 HMFCA 的形成。对 WPP 测试了 HMF 至 FDCA 催化溶剂,在 130 °C 下 120 分钟内,FDCA 总产率为 14.6%。此外,MnFe 由 MnO、MnO、MnO、FeO 和 FeO 的混合相组成,并且表现出 Mn (Mn↔Mn) 和 Fe (Fe↔Fe) 物质氧化态的变化。



























 京公网安备 11010802027423号
京公网安备 11010802027423号