当前位置:
X-MOL 学术
›
J. Hazard. Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Competitive sorption experiments reveal new regression models to predict PhACs sorption on carbonaceous materials
Journal of Hazardous Materials ( IF 13.6 ) Pub Date : 2024-04-16 , DOI: 10.1016/j.jhazmat.2024.134239 Edinsson Muñoz-Vega , Marcel Horovitz , Lisa Dönges , Thomas Schiedek , Stephan Schulz , Christoph Schüth
Journal of Hazardous Materials ( IF 13.6 ) Pub Date : 2024-04-16 , DOI: 10.1016/j.jhazmat.2024.134239 Edinsson Muñoz-Vega , Marcel Horovitz , Lisa Dönges , Thomas Schiedek , Stephan Schulz , Christoph Schüth
|
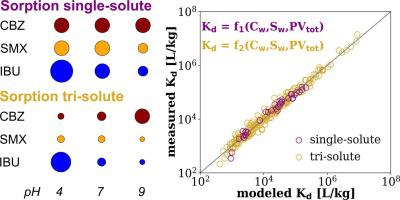
Sorption of hydrophobic organic contaminants onto thermally altered carbonaceous materials (TACM) constitutes a widely used technology for remediation of polluted waters. This process is typically described by sorption isotherms, with one of the most used models, the Polanyi-Dubinin-Manes (PDM) equation, including water solubility () as a normalizing factor. In case of pharmaceutical active compounds (PhACs), depends on the pH of the environment due to the ionic/ionizable behavior of these chemicals, a fact frequently ignored in sorption studies of PhACs. In this work, we set the theoretical framework to include the variation of with pH in the definition of the PDM model, and we applied this approach to describe the effect of ambient pH in the competitive sorption of three commonly detected PhACs (carbamazepine, ibuprofen, and sulfamethoxazole) onto three carbonaceous sorbents (biochar, powder activated carbon, and colloidal activated carbon). Changes in the ambient pH and hence in the hydrophobicity of the compounds could explain the strong variations observed in single-solute sorption and also in competitive sorption. Furthermore, was used as a parameter for the linear regression model of sorption coefficients of our experiments, suggesting the incorporation of this variable as an improvement to existing approaches for prediction of PhACs sorption onto TACM.
中文翻译:

竞争性吸附实验揭示了预测 PhAC 在碳质材料上吸附的新回归模型
将疏水性有机污染物吸附到热变碳质材料(TACM)上是一种广泛使用的污染水体修复技术。该过程通常通过吸附等温线来描述,其中最常用的模型之一是 Polanyi-Dubinin-Manes (PDM) 方程,其中包括水溶性 () 作为归一化因子。对于药物活性化合物 (PhAC),由于这些化学物质的离子/电离行为,取决于环境的 pH 值,这一事实在 PhAC 的吸附研究中经常被忽视。在这项工作中,我们设定了理论框架,在 PDM 模型的定义中包括随 pH 值的变化,并应用这种方法来描述环境 pH 值对三种常见检测到的 PhAC(卡马西平、布洛芬、和磺胺甲恶唑)到三种碳质吸附剂(生物炭、粉末活性炭和胶体活性炭)上。环境 pH 值的变化以及化合物疏水性的变化可以解释在单溶质吸附和竞争吸附中观察到的强烈变化。此外, 被用作我们实验的吸附系数的线性回归模型的参数,表明该变量的合并作为对 TACM 上 PhAC 吸附预测的现有方法的改进。
更新日期:2024-04-16
中文翻译:

竞争性吸附实验揭示了预测 PhAC 在碳质材料上吸附的新回归模型
将疏水性有机污染物吸附到热变碳质材料(TACM)上是一种广泛使用的污染水体修复技术。该过程通常通过吸附等温线来描述,其中最常用的模型之一是 Polanyi-Dubinin-Manes (PDM) 方程,其中包括水溶性 () 作为归一化因子。对于药物活性化合物 (PhAC),由于这些化学物质的离子/电离行为,取决于环境的 pH 值,这一事实在 PhAC 的吸附研究中经常被忽视。在这项工作中,我们设定了理论框架,在 PDM 模型的定义中包括随 pH 值的变化,并应用这种方法来描述环境 pH 值对三种常见检测到的 PhAC(卡马西平、布洛芬、和磺胺甲恶唑)到三种碳质吸附剂(生物炭、粉末活性炭和胶体活性炭)上。环境 pH 值的变化以及化合物疏水性的变化可以解释在单溶质吸附和竞争吸附中观察到的强烈变化。此外, 被用作我们实验的吸附系数的线性回归模型的参数,表明该变量的合并作为对 TACM 上 PhAC 吸附预测的现有方法的改进。



























 京公网安备 11010802027423号
京公网安备 11010802027423号