当前位置:
X-MOL 学术
›
Chem. Eng. J.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Ferrate(VI)-based synergistic oxidation processes (Fe(VI)-SOPs): Promoted reactive species production, micropollutant/microorganism elimination, and toxicity reduction
Chemical Engineering Journal ( IF 15.1 ) Pub Date : 2024-04-16 , DOI: 10.1016/j.cej.2024.151180 Jie-Yu Cao , Ye Du , Xin Dai , Tong Liu , Zhong-Juan Wang , Jie Li , Heng Zhang , Peng Zhou , Bo Lai
Chemical Engineering Journal ( IF 15.1 ) Pub Date : 2024-04-16 , DOI: 10.1016/j.cej.2024.151180 Jie-Yu Cao , Ye Du , Xin Dai , Tong Liu , Zhong-Juan Wang , Jie Li , Heng Zhang , Peng Zhou , Bo Lai
|
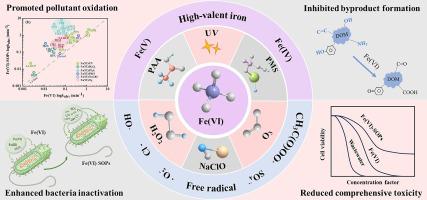
Studies have proposed diverse strategies to activate ferrate(VI) (Fe(VI)) to compensate for the deficiencies of Fe(VI) alone oxidation. This review elucidates the reaction mechanisms of Fe(VI)-based synergistic oxidation processes (Fe(VI)-SOPs), including Fe(VI)/NaClO, Fe(VI)/HO, Fe(VI)/PMS, Fe(VI)/O, Fe(VI)/PAA and Fe(VI)/UV processes. Compared to Fe(VI) alone, Fe(VI)-SOPs provide additional advantages in terms of reactive oxygen species, reactive iron species, and other free radicals actively participating in the oxidation process. In addition, micropollutant oxidation, e.g., ·OH in Fe(VI)/HO and Fe(VI)/O, ·OH and in Fe(VI)/PMS, in Fe(VI)/UV, and the organic free radical in Fe(VI)/PAA. This leads to an enhanced removal rates of micropollutants and the mineralization of TOC. The reaction rate constant of Fe(VI)/PAA was increased by a maximum 143.8 times. Fe(VI)-SOPs create more active sites and pathways for micropollutant oxidation, generally forming more hydroxylation products, thereby facilitating the electron-deficient pollutants to be attacked by high-valent iron. The rate constants for pollutants containing electron-deficient groups can be increased by 1.4 to 143.8 times, thus compensating for the limited oxidation capability of electron-deficient pollutants solely through Fe(VI). Furthermore, Fe(VI)-SOPs exhibited a higher bacterial inactivation capacity but a lower production of byproducts, via the pathways of quenching reactive halogen and promoting precursor oxidation. Additionally, Fe(VI)-SOPs exhibit stronger detoxification effects on comprehensive wastewater toxicity and transformation products of micropollutants by effectively destroying highly toxic groups. This work offers guidance for future research and applications of Fe(VI)-SOPs.
中文翻译:

基于高铁酸盐 (VI) 的协同氧化过程 (Fe(VI)-SOP):促进活性物质的产生、微污染物/微生物的消除和毒性的降低
研究提出了多种激活高铁酸盐 (VI) (Fe(VI)) 的策略,以弥补 Fe(VI) 单独氧化的缺陷。该综述阐明了基于Fe(VI)的协同氧化过程(Fe(VI)-SOPs)的反应机理,包括Fe(VI)/NaClO、Fe(VI)/H2O、Fe(VI)/PMS、Fe(VI)/PMS )/O、Fe(VI)/PAA 和 Fe(VI)/UV 工艺。与单独的 Fe(VI) 相比,Fe(VI)-SOP 在活性氧、活性铁和其他积极参与氧化过程的自由基方面具有额外的优势。此外,微污染物氧化,例如Fe(VI)/H2O和Fe(VI)/O中的·OH,Fe(VI)/PMS中的·OH,Fe(VI)/UV中的·OH,以及Fe(VI)/UV中的有机自由基Fe(VI)/PAA。这会提高微污染物的去除率和 TOC 的矿化。 Fe(VI)/PAA的反应速率常数最大提高了143.8倍。 Fe(VI)-SOPs为微污染物氧化创造了更多的活性位点和途径,通常会形成更多的羟基化产物,从而促进缺电子污染物被高价铁攻击。含有缺电子基团的污染物的速率常数可提高1.4至143.8倍,从而仅通过Fe(VI)补偿缺电子污染物的有限氧化能力。此外,Fe(VI)-SOPs 通过猝灭活性卤素和促进前体氧化的途径表现出较高的细菌灭活能力,但副产物的产生较低。此外,Fe(VI)-SOPs通过有效破坏高毒性基团,对废水综合毒性和微污染物转化产物表现出更强的解毒作用。这项工作为 Fe(VI)-SOP 的未来研究和应用提供了指导。
更新日期:2024-04-16
中文翻译:

基于高铁酸盐 (VI) 的协同氧化过程 (Fe(VI)-SOP):促进活性物质的产生、微污染物/微生物的消除和毒性的降低
研究提出了多种激活高铁酸盐 (VI) (Fe(VI)) 的策略,以弥补 Fe(VI) 单独氧化的缺陷。该综述阐明了基于Fe(VI)的协同氧化过程(Fe(VI)-SOPs)的反应机理,包括Fe(VI)/NaClO、Fe(VI)/H2O、Fe(VI)/PMS、Fe(VI)/PMS )/O、Fe(VI)/PAA 和 Fe(VI)/UV 工艺。与单独的 Fe(VI) 相比,Fe(VI)-SOP 在活性氧、活性铁和其他积极参与氧化过程的自由基方面具有额外的优势。此外,微污染物氧化,例如Fe(VI)/H2O和Fe(VI)/O中的·OH,Fe(VI)/PMS中的·OH,Fe(VI)/UV中的·OH,以及Fe(VI)/UV中的有机自由基Fe(VI)/PAA。这会提高微污染物的去除率和 TOC 的矿化。 Fe(VI)/PAA的反应速率常数最大提高了143.8倍。 Fe(VI)-SOPs为微污染物氧化创造了更多的活性位点和途径,通常会形成更多的羟基化产物,从而促进缺电子污染物被高价铁攻击。含有缺电子基团的污染物的速率常数可提高1.4至143.8倍,从而仅通过Fe(VI)补偿缺电子污染物的有限氧化能力。此外,Fe(VI)-SOPs 通过猝灭活性卤素和促进前体氧化的途径表现出较高的细菌灭活能力,但副产物的产生较低。此外,Fe(VI)-SOPs通过有效破坏高毒性基团,对废水综合毒性和微污染物转化产物表现出更强的解毒作用。这项工作为 Fe(VI)-SOP 的未来研究和应用提供了指导。



























 京公网安备 11010802027423号
京公网安备 11010802027423号