Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Molecular Determinant Underlying Selective Coupling of Primary G‐Protein by Class A GPCRs
Advanced Science ( IF 15.1 ) Pub Date : 2024-04-22 , DOI: 10.1002/advs.202310120 Qingya Shen 1, 2 , Xinyan Tang 3 , Xin Wen 4, 5 , Shizhuo Cheng 1, 2, 6 , Peng Xiao 4, 5 , Shao‐Kun Zang 1, 2 , Dan‐Dan Shen 1, 2 , Lei Jiang 3 , Yanrong Zheng 7 , Huibing Zhang 1, 2 , Haomang Xu 1, 2 , Chunyou Mao 1, 8, 9 , Min Zhang 6 , Weiwei Hu 3 , Jin‐Peng Sun 4, 5, 10 , Yan Zhang 1, 2 , Zhong Chen 3, 7
Advanced Science ( IF 15.1 ) Pub Date : 2024-04-22 , DOI: 10.1002/advs.202310120 Qingya Shen 1, 2 , Xinyan Tang 3 , Xin Wen 4, 5 , Shizhuo Cheng 1, 2, 6 , Peng Xiao 4, 5 , Shao‐Kun Zang 1, 2 , Dan‐Dan Shen 1, 2 , Lei Jiang 3 , Yanrong Zheng 7 , Huibing Zhang 1, 2 , Haomang Xu 1, 2 , Chunyou Mao 1, 8, 9 , Min Zhang 6 , Weiwei Hu 3 , Jin‐Peng Sun 4, 5, 10 , Yan Zhang 1, 2 , Zhong Chen 3, 7
Affiliation
|
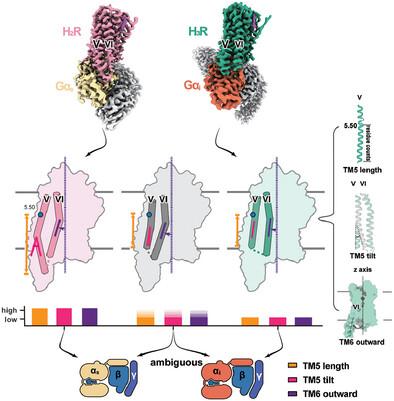
G‐protein‐coupled receptors (GPCRs) transmit downstream signals predominantly via G‐protein pathways. However, the conformational basis of selective coupling of primary G‐protein remains elusive. Histamine receptors H2 R and H3 R couple with Gs ‐ or Gi ‐proteins respectively. Here, three cryo‐EM structures of H2 R‐Gs and H3 R‐Gi complexes are presented at a global resolution of 2.6‐2.7 Å. These structures reveal the unique binding pose for endogenous histamine in H3 R, wherein the amino group interacts with E2065.46 of H3 R instead of the conserved D1143.32 of other aminergic receptors. Furthermore, comparative analysis of the H2 R‐Gs and H3 R‐Gi complexes reveals that the structural geometry of TM5/TM6 determines the primary G‐protein selectivity in histamine receptors. Machine learning (ML)‐based structuromic profiling and functional analysis of class A GPCR–G‐protein complexes illustrate that TM5 length, TM5 tilt, and TM6 outward movement are key determinants of the Gs and Gi/o selectivity among the whole Class A family. Collectively, the findings uncover the common structural geometry within class A GPCRs that determines the primary Gs ‐ and Gi/o ‐coupling selectivity.
中文翻译:

A 类 GPCR 选择性偶联初级 G 蛋白的分子决定因素
G 蛋白偶联受体 (GPCR) 主要通过 G 蛋白途径传递下游信号。然而,初级 G 蛋白选择性偶联的构象基础仍然难以捉摸。组胺受体 H2 R和H3 R 与 G 配对s - 或 G我 分别为-蛋白质。这里,H的三个冷冻电镜结构2 R-Gs 和H3 R-G我 复合物的整体分辨率为 2.6-2.7 Å。这些结构揭示了 H 中内源性组胺的独特结合姿势3 R,其中氨基与E206相互作用5.46 的 H3 R代替保守的D1143.32 其他胺能受体。此外,H的比较分析2 R-Gs 和H3 R-G我 复合物揭示TM5/TM6的结构几何形状决定组胺受体中的主要G蛋白选择性。基于机器学习 (ML) 的 A 类 GPCR-G 蛋白复合物的结构组学分析和功能分析表明,TM5 长度、TM5 倾斜和 TM6 向外运动是 G 的关键决定因素。s 和G输入/输出 整个A类家族之间的选择性。总的来说,这些发现揭示了 A 类 GPCR 中决定主要 G 的常见结构几何形状。s ‐ 和 G输入/输出 ‐耦合选择性。
更新日期:2024-04-22
中文翻译:

A 类 GPCR 选择性偶联初级 G 蛋白的分子决定因素
G 蛋白偶联受体 (GPCR) 主要通过 G 蛋白途径传递下游信号。然而,初级 G 蛋白选择性偶联的构象基础仍然难以捉摸。组胺受体 H



























 京公网安备 11010802027423号
京公网安备 11010802027423号