当前位置:
X-MOL 学术
›
Aliment. Pharm. Ther.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Randomised clinical trial: First‐line infliximab biosimilar is cost‐effective compared to conventional treatment in paediatric Crohn's disease
Alimentary Pharmacology & Therapeutics ( IF 7.6 ) Pub Date : 2024-04-22 , DOI: 10.1111/apt.18000 Stephanie A. Vuijk 1 , Maria M. E. Jongsma 1 , Britt M. Hoeven 1 , Maarten A. Cozijnsen 1 , Merel van Pieterson 1 , Tim G. J. de Meij 2 , Obbe F. Norbruis 3 , Michael Groeneweg 4 , Victorien M. Wolters 5 , Herbert van Wering 6 , Thalia Hummel 7 , Janneke Stapelbroek 8 , Cathelijne van der Feen 9 , Patrick F. van Rheenen 10 , Michiel P. van Wijk 2 , Sarah Teklenburg 3 , Dimitris Rizopoulos 11, 12 , Marten J. Poley 13, 14 , Johanna C. Escher 1 , Lissy de Ridder 1
Alimentary Pharmacology & Therapeutics ( IF 7.6 ) Pub Date : 2024-04-22 , DOI: 10.1111/apt.18000 Stephanie A. Vuijk 1 , Maria M. E. Jongsma 1 , Britt M. Hoeven 1 , Maarten A. Cozijnsen 1 , Merel van Pieterson 1 , Tim G. J. de Meij 2 , Obbe F. Norbruis 3 , Michael Groeneweg 4 , Victorien M. Wolters 5 , Herbert van Wering 6 , Thalia Hummel 7 , Janneke Stapelbroek 8 , Cathelijne van der Feen 9 , Patrick F. van Rheenen 10 , Michiel P. van Wijk 2 , Sarah Teklenburg 3 , Dimitris Rizopoulos 11, 12 , Marten J. Poley 13, 14 , Johanna C. Escher 1 , Lissy de Ridder 1
Affiliation
|
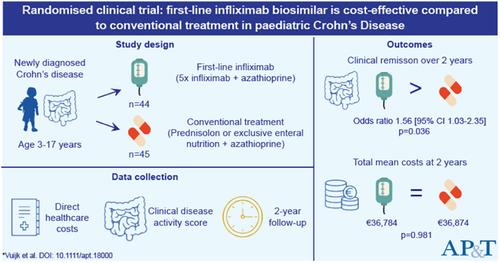
SummaryBackgroundData on cost‐effectiveness of first‐line infliximab in paediatric patients with Crohn's disease are limited. Since biologics are increasingly prescribed and accompanied by high costs, this knowledge gap needs to be addressed.AimTo investigate the cost‐effectiveness of first‐line infliximab compared to conventional treatment in children with moderate‐to‐severe Crohn's disease.MethodsWe included patients from the Top‐down Infliximab Study in Kids with Crohn's disease randomised controlled trial. Children with newly diagnosed moderate‐to‐severe Crohn's disease were treated with azathioprine maintenance and either five induction infliximab (biosimilar) infusions or conventional induction treatment (exclusive enteral nutrition or corticosteroids). Direct healthcare consumption and costs were obtained per patient until week 104. This included data on outpatient hospital visits, hospital admissions, drug costs, endoscopies and surgeries. The primary health outcome was the odds ratio of being in clinical remission (weighted paediatric Crohn's disease activity index<12.5) during 104 weeks.ResultsWe included 89 patients (44 in the first‐line infliximab group and 45 in the conventional treatment group). Mean direct healthcare costs per patient were €36,784 for first‐line infliximab treatment and €36,874 for conventional treatment over 2 years (p = 0.981). The odds ratio of first‐line infliximab versus conventional treatment to be in clinical remission over 104 weeks was 1.56 (95%CI 1.03–2.35, p = 0.036).ConclusionsFirst‐line infliximab treatment resulted in higher odds of being in clinical remission without being more expensive, making it the dominant strategy over conventional treatment in the first 2 years after diagnosis in children with moderate‐to‐severe Crohn's disease.Trial registration number: NCT02517684.
中文翻译:

随机临床试验:与常规治疗儿童克罗恩病相比,一线英夫利昔单抗生物仿制药具有成本效益
摘要背景有关克罗恩病儿科患者一线英夫利昔单抗成本效益的数据有限。由于生物制剂越来越多地开处方且伴随着高昂的成本,因此需要解决这一知识差距。目的是研究一线英夫利昔单抗与常规治疗相比在中重度克罗恩病儿童中的成本效益。方法我们纳入了来自克罗恩病儿童自上而下的英夫利昔单抗研究随机对照试验。新诊断的中重度克罗恩病儿童接受硫唑嘌呤维持治疗和五次英夫利昔单抗(生物仿制药)诱导输注或常规诱导治疗(仅肠内营养或皮质类固醇)。在第 104 周之前,我们获取了每位患者的直接医疗保健消费和成本。这包括门诊就诊、入院、药品成本、内窥镜检查和手术的数据。主要健康结局是 104 周内临床缓解的比值比(加权儿科克罗恩病活动指数<12.5)。结果我们纳入了 89 名患者(一线英夫利昔单抗组 44 名,常规治疗组 45 名)。每位患者的一线英夫利昔单抗治疗平均直接医疗费用为 36,784 欧元,两年以上常规治疗为 36,874 欧元(p = 0.981)。一线英夫利昔单抗与常规治疗在 104 周内达到临床缓解的比值比为 1.56(95% CI 1.03-2.35,p = 0.036)。结论一线英夫利昔单抗治疗在不增加费用的情况下获得临床缓解的几率更高,使其成为中重度克罗恩病儿童诊断后头 2 年内相对于常规治疗的主导策略。试验注册号:NCT02517684。
更新日期:2024-04-22
中文翻译:

随机临床试验:与常规治疗儿童克罗恩病相比,一线英夫利昔单抗生物仿制药具有成本效益
摘要背景有关克罗恩病儿科患者一线英夫利昔单抗成本效益的数据有限。由于生物制剂越来越多地开处方且伴随着高昂的成本,因此需要解决这一知识差距。目的是研究一线英夫利昔单抗与常规治疗相比在中重度克罗恩病儿童中的成本效益。方法我们纳入了来自克罗恩病儿童自上而下的英夫利昔单抗研究随机对照试验。新诊断的中重度克罗恩病儿童接受硫唑嘌呤维持治疗和五次英夫利昔单抗(生物仿制药)诱导输注或常规诱导治疗(仅肠内营养或皮质类固醇)。在第 104 周之前,我们获取了每位患者的直接医疗保健消费和成本。这包括门诊就诊、入院、药品成本、内窥镜检查和手术的数据。主要健康结局是 104 周内临床缓解的比值比(加权儿科克罗恩病活动指数<12.5)。结果我们纳入了 89 名患者(一线英夫利昔单抗组 44 名,常规治疗组 45 名)。每位患者的一线英夫利昔单抗治疗平均直接医疗费用为 36,784 欧元,两年以上常规治疗为 36,874 欧元(



























 京公网安备 11010802027423号
京公网安备 11010802027423号