当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Suppressing Metal Leaching and Sintering in Hydroformylation Reaction by Modulating the Coordination of Rh Single Atoms with Reactants
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2024-04-19 , DOI: 10.1021/jacs.4c01315 Zhounan Yu 1, 2 , Shengxin Zhang 1, 2 , Leilei Zhang 1 , Xiaoyan Liu 1 , Zhenghao Jia 1, 3 , Lin Li 1, 3 , Na Ta 1, 3 , An Wang 1, 2 , Wei Liu 1, 3 , Aiqin Wang 1, 3 , Tao Zhang 1, 3
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2024-04-19 , DOI: 10.1021/jacs.4c01315 Zhounan Yu 1, 2 , Shengxin Zhang 1, 2 , Leilei Zhang 1 , Xiaoyan Liu 1 , Zhenghao Jia 1, 3 , Lin Li 1, 3 , Na Ta 1, 3 , An Wang 1, 2 , Wei Liu 1, 3 , Aiqin Wang 1, 3 , Tao Zhang 1, 3
Affiliation
|
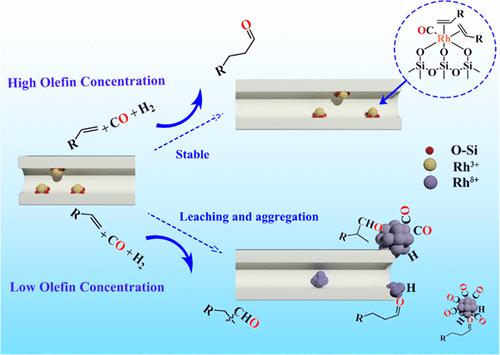
Hydroformylation reaction is one of the largest homogeneously catalyzed industrial processes yet suffers from difficulty and high cost in catalyst separation and recovery. Heterogeneous single-atom catalysts (SACs), on the other hand, have emerged as a promising alternative due to their high initial activity and reasonable regioselectivity. Nevertheless, the stability of SACs against metal aggregation and leaching during the reaction has rarely been addressed. Herein, we elucidate the mechanism of Rh aggregation and leaching by investigating the structural evolution of Rh1@silicalite-1 SAC in response to different adsorbates (CO, H2, alkene, and aldehydes) by using diffuse reflectance infrared Fourier transform spectroscopy, X-ray adsorption fine structure, and scanning transmission electron microscopy techniques and kinetic studies. It is discovered that the aggregation and leaching of Rh are induced by the strong adsorption of CO and aldehydes on Rh, as well as the reduction of Rh3+ by CO/H2 which weakens the binding of Rh with support. In contrast, alkene effectively counteracts this effect by the competitive adsorption on Rh atoms with CO/aldehyde, and the disintegration of Rh clusters. Based on these results, we propose a strategy to conduct the reaction under conditions of high alkene concentration, which proves to be able to stabilize Rh single atom against aggregation and/or leaching for more than 100 h time-on-stream.
中文翻译:

通过调节Rh单原子与反应物的配位来抑制加氢甲酰化反应中的金属浸出和烧结
加氢甲酰化反应是最大的均相催化工业过程之一,但催化剂分离和回收困难且成本高。另一方面,多相单原子催化剂(SAC)由于其高初始活性和合理的区域选择性而成为一种有前途的替代品。然而,SAC 在反应过程中抵抗金属聚集和浸出的稳定性很少得到解决。在此,我们通过使用漫反射红外傅里叶变换光谱研究Rh 1 @silicalite-1 SAC响应不同吸附物(CO、H 2 、烯烃和醛)的结构演化,阐明了Rh聚集和浸出的机制,X射线吸附精细结构,以及扫描透射电子显微镜技术和动力学研究。研究发现Rh的聚集和浸出是由于CO和醛类对Rh的强烈吸附以及CO/H 2对Rh 3+的还原削弱了Rh与载体的结合而引起的。相比之下,烯烃通过 CO/醛在 Rh 原子上的竞争吸附以及 Rh 簇的分解,有效地抵消了这种效应。基于这些结果,我们提出了一种在高烯烃浓度条件下进行反应的策略,该策略被证明能够稳定 Rh 单原子,防止聚集和/或浸出,运行时间超过 100 小时。
更新日期:2024-04-19
中文翻译:

通过调节Rh单原子与反应物的配位来抑制加氢甲酰化反应中的金属浸出和烧结
加氢甲酰化反应是最大的均相催化工业过程之一,但催化剂分离和回收困难且成本高。另一方面,多相单原子催化剂(SAC)由于其高初始活性和合理的区域选择性而成为一种有前途的替代品。然而,SAC 在反应过程中抵抗金属聚集和浸出的稳定性很少得到解决。在此,我们通过使用漫反射红外傅里叶变换光谱研究Rh 1 @silicalite-1 SAC响应不同吸附物(CO、H 2 、烯烃和醛)的结构演化,阐明了Rh聚集和浸出的机制,X射线吸附精细结构,以及扫描透射电子显微镜技术和动力学研究。研究发现Rh的聚集和浸出是由于CO和醛类对Rh的强烈吸附以及CO/H 2对Rh 3+的还原削弱了Rh与载体的结合而引起的。相比之下,烯烃通过 CO/醛在 Rh 原子上的竞争吸附以及 Rh 簇的分解,有效地抵消了这种效应。基于这些结果,我们提出了一种在高烯烃浓度条件下进行反应的策略,该策略被证明能够稳定 Rh 单原子,防止聚集和/或浸出,运行时间超过 100 小时。



























 京公网安备 11010802027423号
京公网安备 11010802027423号