当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Can the Rate of a Catalytic Turnover Be Altered by Ligands in the Absence of Direct Binding Interactions?
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2024-04-22 , DOI: 10.1021/jacs.4c01978 Jacklyn N. Hall 1, 2 , Stephen P. Vicchio 3 , A. Jeremy Kropf 2 , Massimiliano Delferro 2 , Praveen Bollini 1
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2024-04-22 , DOI: 10.1021/jacs.4c01978 Jacklyn N. Hall 1, 2 , Stephen P. Vicchio 3 , A. Jeremy Kropf 2 , Massimiliano Delferro 2 , Praveen Bollini 1
Affiliation
|
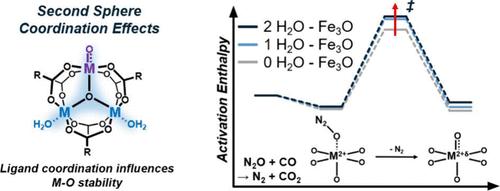
Second sphere coordination effects ubiquitous in enzymatic catalysis occur through direct interactions, either covalent or non-covalent, with reaction intermediates and transition states. We present herein evidence of indirect second sphere coordination effects in which ligation of water/alkanols far removed from the primary coordination sphere of the active site nevertheless alter energetic landscapes within catalytic redox cycles in the absence of direct physicochemical interactions with surface species mediating catalytic turnovers. Density functional theory, in situ X-ray absorption and infrared spectroscopy, and a wide array of steady-state and transient CO oxidation rate data suggest that the presence of peripheral water renders oxidation half-cycles within two-electron redox cycles over μ3-oxo-bridged trimers in MIL-100(M) more kinetically demanding. Communication between ligated water and the active site appears to occur through the Fe–O–Fe backbone, as inferred from spin density variations on the central μ3-oxygen ‘junction’. Evidence is provided for the generality of these second sphere effects in that they influence different types of redox half-cycles or metals, and can be amplified or attenuated through choice of coordinating ligand. Specifically in the case of MIL-100(M) materials, the Cr isostructure can be made to kinetically mimic the Fe variant by disproportionately hindering oxidation half-cycles relative to the reduction half-cycles. Kinetic and spectroscopic inferences presented here significantly expand both the conceptual definition of second sphere effects as well as the palette of synthetic levers available for tuning catalytic redox performance through chemical ligation.
中文翻译:

在没有直接结合相互作用的情况下,配体可以改变催化转化率吗?
酶催化中普遍存在的第二球配位效应是通过与反应中间体和过渡态的直接相互作用(共价或非共价)发生的。我们在此提出了间接第二球配位效应的证据,其中远离活性位点的主要配位球的水/烷醇的连接在不存在与介导催化周转的表面物种的直接物理化学相互作用的情况下改变了催化氧化还原循环内的能量景观。密度泛函理论、原位 X 射线吸收和红外光谱以及大量稳态和瞬态 CO 氧化速率数据表明,外围水的存在导致 μ 3 -上双电子氧化还原循环内的氧化半循环MIL-100(M) 中的氧桥三聚体对动力学的要求更高。连接水和活性位点之间的通讯似乎是通过 Fe-O-Fe 主链发生的,正如从中心 μ 3 -氧“连接点”上的自旋密度变化推断的那样。提供了证据证明这些第二球体效应的普遍性,因为它们影响不同类型的氧化还原半循环或金属,并且可以通过选择配位配体来放大或减弱。特别是在 MIL-100(M) 材料的情况下,可以通过相对于还原半循环不成比例地阻碍氧化半循环,使 Cr 同工结构在动力学上模拟 Fe 变体。这里提出的动力学和光谱推论显着扩展了第二球体效应的概念定义以及可用于通过化学连接调节催化氧化还原性能的合成杠杆的调色板。
更新日期:2024-04-22
中文翻译:

在没有直接结合相互作用的情况下,配体可以改变催化转化率吗?
酶催化中普遍存在的第二球配位效应是通过与反应中间体和过渡态的直接相互作用(共价或非共价)发生的。我们在此提出了间接第二球配位效应的证据,其中远离活性位点的主要配位球的水/烷醇的连接在不存在与介导催化周转的表面物种的直接物理化学相互作用的情况下改变了催化氧化还原循环内的能量景观。密度泛函理论、原位 X 射线吸收和红外光谱以及大量稳态和瞬态 CO 氧化速率数据表明,外围水的存在导致 μ 3 -上双电子氧化还原循环内的氧化半循环MIL-100(M) 中的氧桥三聚体对动力学的要求更高。连接水和活性位点之间的通讯似乎是通过 Fe-O-Fe 主链发生的,正如从中心 μ 3 -氧“连接点”上的自旋密度变化推断的那样。提供了证据证明这些第二球体效应的普遍性,因为它们影响不同类型的氧化还原半循环或金属,并且可以通过选择配位配体来放大或减弱。特别是在 MIL-100(M) 材料的情况下,可以通过相对于还原半循环不成比例地阻碍氧化半循环,使 Cr 同工结构在动力学上模拟 Fe 变体。这里提出的动力学和光谱推论显着扩展了第二球体效应的概念定义以及可用于通过化学连接调节催化氧化还原性能的合成杠杆的调色板。



























 京公网安备 11010802027423号
京公网安备 11010802027423号