当前位置:
X-MOL 学术
›
ACS Appl. Mater. Interfaces
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Enhancing Phototoxicity in Human Colorectal Tumor Cells Through Nanoarchitectonics for Synergistic Photothermal and Photodynamic Therapies
ACS Applied Materials & Interfaces ( IF 9.5 ) Pub Date : 2024-04-23 , DOI: 10.1021/acsami.4c02247 Alexandre Mendes de Almeida Junior 1 , André Satoshi Ferreira 1 , Sabrina Aléssio Camacho 1, 2 , Lucas Gontijo Moreira 1 , Karina Alves de Toledo 1 , Osvaldo N. Oliveira 2 , Pedro Henrique Benites Aoki 1
ACS Applied Materials & Interfaces ( IF 9.5 ) Pub Date : 2024-04-23 , DOI: 10.1021/acsami.4c02247 Alexandre Mendes de Almeida Junior 1 , André Satoshi Ferreira 1 , Sabrina Aléssio Camacho 1, 2 , Lucas Gontijo Moreira 1 , Karina Alves de Toledo 1 , Osvaldo N. Oliveira 2 , Pedro Henrique Benites Aoki 1
Affiliation
|
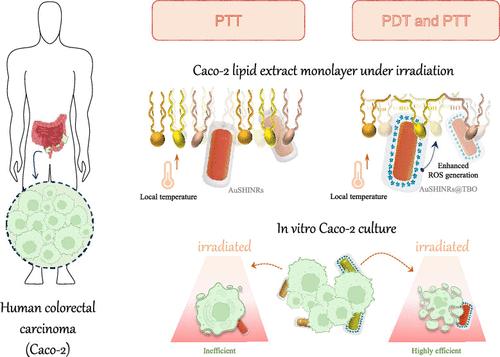
Phototherapies are promising for noninvasive treatment of aggressive tumors, especially when combining heat induction and oxidative processes. Herein, we show enhanced phototoxicity of gold shell-isolated nanorods conjugated with toluidine blue-O (AuSHINRs@TBO) against human colorectal tumor cells (Caco-2) with synergic effects of photothermal (PTT) and photodynamic therapies (PDT). Mitochondrial metabolic activity tests (MTT) performed on Caco-2 cell cultures indicated a photothermal effect from AuSHINRs owing to enhanced light absorption from the localized surface plasmon resonance (LSPR). The phototoxicity against Caco-2 cells was further increased with AuSHINRs@TBO where oxidative processes, such as hydroperoxidation, were also present, leading to a cell viability reduction from 85.5 to 39.0%. The molecular-level mechanisms responsible for these effects were investigated on bioinspired tumor membranes using Langmuir monolayers of Caco-2 lipid extract. Polarization-modulation infrared reflection–absorption spectroscopy (PM-IRRAS) revealed that the AuSHINRs@TBO incorporation is due to attractive electrostatic interactions with negatively charged groups of the Caco-2 lipid extract, resulting in the expansion of surface pressure isotherms. Upon irradiation, Caco-2 lipid extract monolayers containing AuSHINRs@TBO (1:1 v/v) exhibited ca. 1.0% increase in surface area. This is attributed to the generation of reactive oxygen species (ROS) and their interaction with Caco-2 lipid extract monolayers, leading to hydroperoxide formation. The oxidative effects are facilitated by AuSHINRs@TBO penetration into the polar groups of the extract, allowing oxidative reactions with carbon chain unsaturations. These mechanisms are consistent with findings from confocal fluorescence microscopy, where the Caco-2 plasma membrane was the primary site of the cell death induction process.
中文翻译:

通过纳米结构增强人类结直肠肿瘤细胞的光毒性以实现协同光热和光动力疗法
光疗法有望用于侵袭性肿瘤的无创治疗,特别是在结合热诱导和氧化过程时。在此,我们展示了与甲苯胺蓝-O (AuSHINRs@TBO) 结合的金壳隔离纳米棒对人结直肠肿瘤细胞 (Caco-2) 的光毒性增强,并具有光热 (PTT) 和光动力疗法 (PDT) 的协同作用。对 Caco-2 细胞培养物进行的线粒体代谢活性测试 (MTT) 表明,由于局部表面等离子共振 (LSPR) 的光吸收增强,AuSHINR 产生光热效应。 AuSHINRs@TBO 进一步增强了对 Caco-2 细胞的光毒性,其中还存在氧化过程,例如氢过氧化,导致细胞活力从 85.5% 降低至 39.0%。使用 Caco-2 脂质提取物的 Langmuir 单层在仿生肿瘤膜上研究了造成这些影响的分子水平机制。偏振调制红外反射吸收光谱 (PM-IRRAS) 显示 AuSHINRs@TBO 的掺入是由于与 Caco-2 脂质提取物的带负电基团的吸引性静电相互作用,导致表面压力等温线的扩展。照射后,含有 AuSHINRs@TBO (1:1 v/v) 的 Caco-2 脂质提取物单层表现出约表面积增加 1.0%。这是由于活性氧 (ROS) 的产生及其与 Caco-2 脂质提取物单层的相互作用,导致氢过氧化物的形成。 AuSHINRs@TBO 渗透到提取物的极性基团中,从而促进与碳链不饱和度的氧化反应,从而促进氧化作用。这些机制与共聚焦荧光显微镜的发现一致,其中 Caco-2 质膜是细胞死亡诱导过程的主要部位。
更新日期:2024-04-24
中文翻译:

通过纳米结构增强人类结直肠肿瘤细胞的光毒性以实现协同光热和光动力疗法
光疗法有望用于侵袭性肿瘤的无创治疗,特别是在结合热诱导和氧化过程时。在此,我们展示了与甲苯胺蓝-O (AuSHINRs@TBO) 结合的金壳隔离纳米棒对人结直肠肿瘤细胞 (Caco-2) 的光毒性增强,并具有光热 (PTT) 和光动力疗法 (PDT) 的协同作用。对 Caco-2 细胞培养物进行的线粒体代谢活性测试 (MTT) 表明,由于局部表面等离子共振 (LSPR) 的光吸收增强,AuSHINR 产生光热效应。 AuSHINRs@TBO 进一步增强了对 Caco-2 细胞的光毒性,其中还存在氧化过程,例如氢过氧化,导致细胞活力从 85.5% 降低至 39.0%。使用 Caco-2 脂质提取物的 Langmuir 单层在仿生肿瘤膜上研究了造成这些影响的分子水平机制。偏振调制红外反射吸收光谱 (PM-IRRAS) 显示 AuSHINRs@TBO 的掺入是由于与 Caco-2 脂质提取物的带负电基团的吸引性静电相互作用,导致表面压力等温线的扩展。照射后,含有 AuSHINRs@TBO (1:1 v/v) 的 Caco-2 脂质提取物单层表现出约表面积增加 1.0%。这是由于活性氧 (ROS) 的产生及其与 Caco-2 脂质提取物单层的相互作用,导致氢过氧化物的形成。 AuSHINRs@TBO 渗透到提取物的极性基团中,从而促进与碳链不饱和度的氧化反应,从而促进氧化作用。这些机制与共聚焦荧光显微镜的发现一致,其中 Caco-2 质膜是细胞死亡诱导过程的主要部位。



























 京公网安备 11010802027423号
京公网安备 11010802027423号