当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Mechanistic Origin of Ligand Effects on Exhaustive Functionalization During Pd-Catalyzed Cross-Coupling of Dihaloarenes
ACS Catalysis ( IF 12.9 ) Pub Date : 2024-04-23 , DOI: 10.1021/acscatal.4c00646 Nathaniel G. Larson 1 , Jacob P. Norman 1 , Sharon R. Neufeldt 1
ACS Catalysis ( IF 12.9 ) Pub Date : 2024-04-23 , DOI: 10.1021/acscatal.4c00646 Nathaniel G. Larson 1 , Jacob P. Norman 1 , Sharon R. Neufeldt 1
Affiliation
|
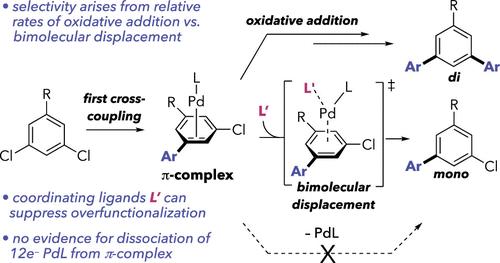
We describe a detailed investigation into why bulky ligands─those that enable catalysis at “12e–” Pd0─tend to promote overfunctionalization during Pd-catalyzed cross-couplings of dihalogenated substrates. After one cross-coupling event takes place, PdL initially remains coordinated to the π system of the nascent product. Selectivity for mono- vs difunctionalization arises from the relative rates of π-decomplexation versus a second oxidative addition. Under the Suzuki coupling conditions in this work, direct dissociation of 12e– PdL from the π-complex cannot outcompete oxidative addition. Instead, Pd must be displaced from the π-complex as 14e– PdL(L’) by a second incoming ligand L’. The incoming ligand is another molecule of dichloroarene if the reaction conditions do not include π-coordinating solvents or additives. More overfunctionalization tends to result when increased ligand or substrate sterics raises the energy of the bimolecular transition state for separating 14e– PdL(L’) from the monocross-coupled product. This work has practical implications for optimizing the selectivity in cross-couplings involving multiple halogens. For example, we demonstrate that small coordinating additives like DMSO can largely suppress overfunctionalization and that the precatalyst structure can also impact selectivity.
中文翻译:

Pd 催化二卤代芳烃交叉偶联过程中配体对详尽功能化影响的机制起源
我们详细研究了为什么大配体(那些能够在“12 e - ” Pd 0处催化的配体)在 Pd 催化的二卤化底物交叉偶联过程中倾向于促进过度功能化。发生一次交叉偶联事件后,PdL 最初仍与新生产物的 π 系统保持配位。单官能化与双官能化的选择性取决于π-解络与第二次氧化加成的相对速率。在这项工作中的 Suzuki 偶联条件下,12 e – PdL 从 π 络合物的直接解离不能胜过氧化加成。相反,Pd 必须被第二个引入的配体 L' 从π 络合物中置换为 14 e – PdL(L')。如果反应条件不包括π配位溶剂或添加剂,则引入的配体是另一个二氯芳烃分子。当配体或底物空间增加时,用于从单交叉偶联产物中分离 14 e – PdL(L')的双分子过渡态能量增加,往往会导致更多的过度官能化。这项工作对于优化涉及多个卤素的交叉偶联的选择性具有实际意义。例如,我们证明像 DMSO 这样的小配位添加剂可以在很大程度上抑制过度官能化,并且预催化剂结构也会影响选择性。
更新日期:2024-04-23
中文翻译:

Pd 催化二卤代芳烃交叉偶联过程中配体对详尽功能化影响的机制起源
我们详细研究了为什么大配体(那些能够在“12 e - ” Pd 0处催化的配体)在 Pd 催化的二卤化底物交叉偶联过程中倾向于促进过度功能化。发生一次交叉偶联事件后,PdL 最初仍与新生产物的 π 系统保持配位。单官能化与双官能化的选择性取决于π-解络与第二次氧化加成的相对速率。在这项工作中的 Suzuki 偶联条件下,12 e – PdL 从 π 络合物的直接解离不能胜过氧化加成。相反,Pd 必须被第二个引入的配体 L' 从π 络合物中置换为 14 e – PdL(L')。如果反应条件不包括π配位溶剂或添加剂,则引入的配体是另一个二氯芳烃分子。当配体或底物空间增加时,用于从单交叉偶联产物中分离 14 e – PdL(L')的双分子过渡态能量增加,往往会导致更多的过度官能化。这项工作对于优化涉及多个卤素的交叉偶联的选择性具有实际意义。例如,我们证明像 DMSO 这样的小配位添加剂可以在很大程度上抑制过度官能化,并且预催化剂结构也会影响选择性。



























 京公网安备 11010802027423号
京公网安备 11010802027423号