当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
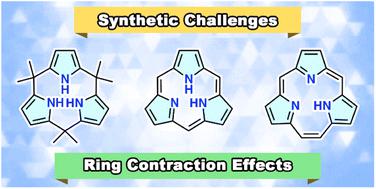
Contracted porphyrins and calixpyrroles: synthetic challenges and ring-contraction effects
Chemical Science ( IF 8.4 ) Pub Date : 2024-04-24 , DOI: 10.1039/d4sc02028f Keita Watanabe 1 , Narendra Nath Pati 2 , Yasuhide Inokuma 1, 2
Chemical Science ( IF 8.4 ) Pub Date : 2024-04-24 , DOI: 10.1039/d4sc02028f Keita Watanabe 1 , Narendra Nath Pati 2 , Yasuhide Inokuma 1, 2
Affiliation
|
Ring-contracted porphyrin analogues, such as subporphyrins and calix[3]pyrroles, have recently attracted considerable attention not only as challenging synthetic targets but also as functional macrocyclic compounds. Although canonical porphyrins and calix[4]pyrrole are selectively generated via acid-catalyzed condensation reactions of pyrrole monomers, their tripyrrolic analogues are always missing under similar conditions. Recent progress in synthesis has shown that strain-controlled approaches using boron(III)-templating, core-modification, or ring tightening provide access to various contracted porphyrins. The tripyrrolic macrocycles are a new class of functional macrocycles exhibiting unique ring-contraction effects, including strong boron chelation and strain-induced ring expansion. This Perspective reviews recent advances in synthetic strategies and the novel ring-contraction effects of subporphyrins, triphyrins(2.1.1), calix[3]pyrroles, and their analogous.
中文翻译:

收缩卟啉和杯吡咯:合成挑战和环收缩效应
缩环卟啉类似物,例如亚卟啉和杯[3]吡咯,最近不仅作为具有挑战性的合成目标,而且作为功能性大环化合物引起了相当大的关注。尽管典型的卟啉和杯[4]吡咯是通过吡咯单体的酸催化缩合反应选择性生成的,但在相似的条件下它们的三吡咯类似物总是缺失。合成方面的最新进展表明,使用硼 ( III ) 模板、核心修饰或环紧缩的应变控制方法可提供各种收缩卟啉。三吡咯大环化合物是一类新型功能性大环化合物,表现出独特的环收缩效应,包括强硼螯合和应变诱导的环扩张。本综述回顾了亚卟啉、三卟啉(2.1.1)、杯[3]吡咯及其类似物的合成策略和新型环收缩效应的最新进展。
更新日期:2024-04-29
中文翻译:

收缩卟啉和杯吡咯:合成挑战和环收缩效应
缩环卟啉类似物,例如亚卟啉和杯[3]吡咯,最近不仅作为具有挑战性的合成目标,而且作为功能性大环化合物引起了相当大的关注。尽管典型的卟啉和杯[4]吡咯是通过吡咯单体的酸催化缩合反应选择性生成的,但在相似的条件下它们的三吡咯类似物总是缺失。合成方面的最新进展表明,使用硼 ( III ) 模板、核心修饰或环紧缩的应变控制方法可提供各种收缩卟啉。三吡咯大环化合物是一类新型功能性大环化合物,表现出独特的环收缩效应,包括强硼螯合和应变诱导的环扩张。本综述回顾了亚卟啉、三卟啉(2.1.1)、杯[3]吡咯及其类似物的合成策略和新型环收缩效应的最新进展。



























 京公网安备 11010802027423号
京公网安备 11010802027423号