当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Unifying N-Sulfinylamines with Alkyltrifluoroborates by Organophotoredox Catalysis: Access to Functionalized Alkylsulfinamides and High-Valent S(VI) Analogues
Organic Letters ( IF 5.2 ) Pub Date : 2024-04-22 , DOI: 10.1021/acs.orglett.4c01270 Subham Das 1 , Pinku Prasad Mondal 1 , Amit Dhibar 1 , Aan Ruth 1 , Basudev Sahoo 1
Organic Letters ( IF 5.2 ) Pub Date : 2024-04-22 , DOI: 10.1021/acs.orglett.4c01270 Subham Das 1 , Pinku Prasad Mondal 1 , Amit Dhibar 1 , Aan Ruth 1 , Basudev Sahoo 1
Affiliation
|
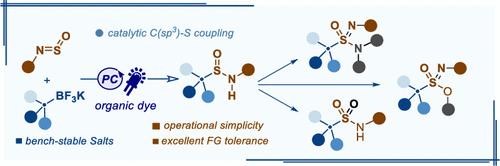
We describe an organophotoredox-catalyzed sp3 C–S coupling of N-sulfinylamines with bench-stable alkyltrifluoroborates as a latent nucleophilic counterpart en route to alkylsulfinamides in high efficiency. In contrast to the two-electron reactivity of traditional organometallic reagents, this catalytic method reports the single-electron process of an organometallic reagent with N-sulfinylamines in C–S coupling. This mild and scalable protocol offers operational simplicity and exceptional functional group compatibility, including ketone, ester, amide, nitrile, and halides, that is vulnerable to organolithium or Grignard reagents. Additionally, the sulfinamides are conveniently converted to a variety of important S(VI) compounds, like sulfonamides, sulfonimidamides, and sulfonimidates, among others.
中文翻译:

通过有机光氧化还原催化将 N-亚磺胺与烷基三氟硼酸盐统一:获得功能化烷基亚磺酰胺和高价 S(VI) 类似物
我们描述了一种有机光氧化还原催化的 sp 3 C-S 偶联,将N -亚磺酰胺与实验室稳定的烷基三氟硼酸盐作为潜在亲核对应物,高效地生成烷基亚磺酰胺。与传统有机金属试剂的双电子反应性相比,该催化方法报道了有机金属试剂与N-亚磺酰胺在C-S偶联中的单电子过程。这种温和且可扩展的方案提供了操作简单性和出色的官能团兼容性,包括酮、酯、酰胺、腈和卤化物,这些官能团容易受到有机锂或格氏试剂的影响。此外,亚磺酰胺可以方便地转化为各种重要的 S(VI) 化合物,如磺酰胺、磺酰亚胺和磺酰亚胺酯等。
更新日期:2024-04-22
中文翻译:

通过有机光氧化还原催化将 N-亚磺胺与烷基三氟硼酸盐统一:获得功能化烷基亚磺酰胺和高价 S(VI) 类似物
我们描述了一种有机光氧化还原催化的 sp 3 C-S 偶联,将N -亚磺酰胺与实验室稳定的烷基三氟硼酸盐作为潜在亲核对应物,高效地生成烷基亚磺酰胺。与传统有机金属试剂的双电子反应性相比,该催化方法报道了有机金属试剂与N-亚磺酰胺在C-S偶联中的单电子过程。这种温和且可扩展的方案提供了操作简单性和出色的官能团兼容性,包括酮、酯、酰胺、腈和卤化物,这些官能团容易受到有机锂或格氏试剂的影响。此外,亚磺酰胺可以方便地转化为各种重要的 S(VI) 化合物,如磺酰胺、磺酰亚胺和磺酰亚胺酯等。



























 京公网安备 11010802027423号
京公网安备 11010802027423号