Carbon Letters ( IF 4.5 ) Pub Date : 2024-04-24 , DOI: 10.1007/s42823-024-00730-4 Muhammad Asim , Akbar Hussain , Meryem Samancı , Naveed Kausar Janjua , Ayşe Bayrakçeken
|
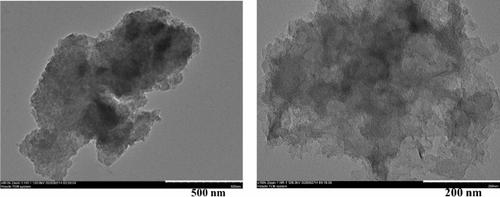
Electrochemical water splitting presents an optimal approach for generating hydrogen (H2), a highly promising alternative energy source. Nevertheless, the slow kinetics of the electrochemical oxygen evolution reaction (OER) and the exorbitant cost, limited availability, and susceptibility to oxidation of noble metal-based electrocatalysts have compelled scientists to investigate cost-effective and efficient electrocatalysts. Bimetallic nanostructured materials have been demonstrated to exhibit improved catalytic performances for the oxygen evolution reaction (OER). Herein, we report carbon aerogel (CA) decorated with different molar ratios of Fe and Ni with enhanced OER activity. Microwave irradiation was involved as a novel strategy during the synthesis process. Inductively coupled plasma mass spectrometry (ICP-MS), X-ray diffraction (XRD), X-ray Photoelectron Spectroscopy (XPS), Scanning Electron Microscope (SEM), Energy dispersive X-ray spectroscopy (EDAX spectra and EDAX mapping), Transmission Electron Microscope (TEM), High-Resolution Transmission Electron Microscope (HR-TEM), and Selected Area Electron Diffraction (SAED) were used for physical characterizations of as-prepared material. Electrochemical potential towards OER was examined through cyclic voltammetry (CV), chronoamperometry, and electrochemical impedance spectroscopy (EIS). The FeNi/CA with optimized molar ratios exhibits low overpotential 377 mV at 10 mAcm−2, smaller Tafel slope (94.5 mV dec−1), and high turnover frequency (1.09 s−1 at 300 mV). Other electrocatalytic parameters were also calculated and compared with previously reported OER catalysts. Additionally, chronoamperometric studies confirmed excellent electrochemical stability, as the OER activity shows minimal change even after a stability test lasting 3600 s. Moreover, the bimetallic (Fe and Ni) carbon aerogel exhibits faster catalytic kinetics and higher conductivity than the monometallic (Fe), which was observed through EIS investigation. This research opens up possibilities for utilizing bi- or multi-metallic anchored carbon aerogel with high conductivities and exceptional electrocatalytic performances in electrochemical energy conversion.
中文翻译:

碳气凝胶负载的 Ni-Fe 催化剂具有优异的析氧反应活性
电化学水分解提供了一种产生氢气(H 2 )的最佳方法,氢气是一种非常有前途的替代能源。然而,电化学析氧反应(OER)的缓慢动力学以及昂贵的成本、有限的可用性以及贵金属基电催化剂对氧化的敏感性,迫使科学家们研究具有成本效益和高效的电催化剂。双金属纳米结构材料已被证明能够改善析氧反应(OER)的催化性能。在此,我们报道了用不同摩尔比的 Fe 和 Ni 修饰的碳气凝胶(CA),具有增强的 OER 活性。微波辐射作为合成过程中的一种新颖策略。电感耦合等离子体质谱 (ICP-MS)、X 射线衍射 (XRD)、X 射线光电子能谱 (XPS)、扫描电子显微镜 (SEM)、能量色散 X 射线光谱(EDAX 光谱和 EDAX 绘图)、透射使用电子显微镜 (TEM)、高分辨率透射电子显微镜 (HR-TEM) 和选区电子衍射 (SAED) 对所制备的材料进行物理表征。通过循环伏安法 (CV)、计时电流法和电化学阻抗谱 (EIS) 检查 OER 的电化学势。具有优化摩尔比的FeNi/CA在10 mAcm -2下表现出低过电势377 mV 、较小的塔菲尔斜率(94.5 mV dec -1)和高周转频率(在300 mV下1.09 s -1 )。还计算了其他电催化参数并与之前报道的 OER 催化剂进行了比较。此外,计时电流研究证实了优异的电化学稳定性,因为即使在持续 3600 秒的稳定性测试后,OER 活性也显示出最小的变化。此外,通过 EIS 研究观察到,双金属(Fe 和 Ni)碳气凝胶比单金属(Fe)表现出更快的催化动力学和更高的电导率。这项研究为在电化学能量转换中利用具有高电导率和卓越电催化性能的双金属或多金属锚定碳气凝胶开辟了可能性。



























 京公网安备 11010802027423号
京公网安备 11010802027423号