Cellular Oncology ( IF 6.6 ) Pub Date : 2024-04-25 , DOI: 10.1007/s13402-024-00945-7 Yangzi Li , Qingya Cui , Sining Liu , Lingling Liu , Megyn Li , Jun Gao , Zheng Li , Wei Cui , Xiaming Zhu , Liqing Kang , Lei Yu , Depei Wu , Xiaowen Tang
|
Purpose
Despite chimeric antigen receptor (CAR) T-cell therapy has achieved great advances in recent year, approximately 50% of relapsed/refractory B cell acute lymphoblastic leukemia (r/r B-ALL) patients treated with CAR-T experience relapse 6 months post CAR-T treatment. CD20 express on 30 to 50% of B-ALL, which makes CD20 Monoclonal Antibody as one of the potential therapy strategies to decrease the tumor burden and improve the efficacy of CAR-T therapy. Adding Rituximab to chemotherapy protocol had been demonstrated to improve the outcome for CD20-positive ALL. However, rare study explored the influence of Rituximab combined with CAR-T therapy.
Methods
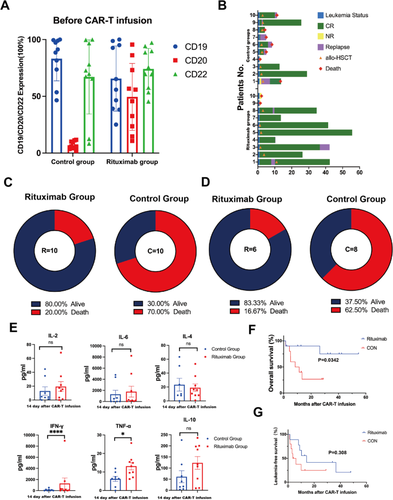
We retrospectively analyzed 20 r/r B-ALL patients who received CAR-T therapy, all of whom had failed multiple lines of therapy. Before CAR-T infusion, we administered Rituximab to 10 patients with high CD20 expression at a dose of 375 mg/m2 for 1 day. Meanwhile, we selected 10 patients with the comparable features who underwent CAR-T treatment without Rituximab in the same period as the control group. In vitro, the surface molecule expression and killing of CAR-T post Rituximab-treated B-ALL cells co-incubated with CAR-T cells were detected by flow cytometry.
Results
The median follow-up of Rituximab and Control groups were 29.27 and 9.83 months. We found that adding Rituximab may confer a favorable prognosis compared with Control group. The 2-year overall survival (OS) and leukemia-free survival (LFS) rates both were longer in the Rituximab group (90% vs. 26.7%, p = 0.0342; 41.7% vs. 25%, p = 0.308). In vitro, we observed that Rituximab-treated tumour cells are more sensitive to CAR-T killing and a broad range of cytokines and chemokines were produced when Rituximab-treated Nalm-6 cells co-cultured with 19-22CAR-T cells, such as interferon-γ (IFN-γ), tumor necrosis factor-α (TNF-α) and interleukin-2 (IL-2). To investigate whether Rituximab has an effect on CAR-T persistence, we stimulated CAR-T cells repeatedly in vitro with Rituximab-treated Nalm-6 to evaluate the changes in CAR-T surface exhaustion molecules at different times. We found that the expression of exhaustion molecules (LAG-3, PD-1, TIM-3) on CAR-T cells were significantly lower in the Rituximab group than in the Control group.
Conclusion
Rituximab combined with CAR-T therapy is effective for improving the long-term prognosis of B-ALL patients who have failed multiple lines of therapy. In vitro, we observed that rituximab potentially improves CAR-T efficacy by sensitizing ALL to CART-mediated cytotoxicity and reducing CAR-T exhaustion.
中文翻译:

利妥昔单抗通过使白血病细胞对 CAR-T 介导的细胞毒性敏感并减少 CAR-T 耗竭,可能改善 CAR-T 疗法治疗 r/r B-ALL 的临床结果
目的
尽管嵌合抗原受体 (CAR) T 细胞疗法近年来取得了巨大进展,但约 50% 的复发/难治性 B 细胞急性淋巴细胞白血病 (r/r B-ALL) 患者在接受 CAR-T 治疗后 6 个月后出现复发CAR-T治疗。 CD20在30%至50%的B-ALL中表达,这使得CD20单克隆抗体成为减轻肿瘤负荷和提高CAR-T疗法疗效的潜在治疗策略之一。已证明在化疗方案中添加利妥昔单抗可改善 CD20 阳性 ALL 的预后。然而,很少有研究探讨利妥昔单抗联合CAR-T治疗的影响。
方法
我们回顾性分析了 20 名接受 CAR-T 治疗的复发/难治 B-ALL 患者,所有患者均多次治疗失败。在CAR-T输注之前,我们给10名高CD20表达的患者注射利妥昔单抗,剂量为375 mg/m 2,持续1天。同时,我们选择了10名具有可比特征且同期接受不使用利妥昔单抗的CAR-T治疗的患者作为对照组。在体外,通过流式细胞仪检测利妥昔单抗处理后与 CAR-T 细胞共孵育的 CAR-T 表面分子表达和杀伤情况。
结果
利妥昔单抗组和对照组的中位随访时间分别为 29.27 个月和 9.83 个月。我们发现,与对照组相比,添加利妥昔单抗可能会带来良好的预后。利妥昔单抗组的 2 年总生存率 (OS) 和无白血病生存率 (LFS) 均较长(90% vs. 26.7%,p = 0.0342;41.7% vs. 25%,p = 0.308)。在体外,我们观察到利妥昔单抗处理的肿瘤细胞对 CAR-T 杀伤更加敏感,并且当利妥昔单抗处理的 Nalm-6 细胞与 19-22CAR-T 细胞共培养时产生广泛的细胞因子和趋化因子,例如干扰素-γ (IFN-γ)、肿瘤坏死因子-α (TNF-α) 和白介素-2 (IL-2)。为了研究利妥昔单抗是否对 CAR-T 持久性有影响,我们用利妥昔单抗处理的 Nalm-6 在体外反复刺激 CAR-T 细胞,以评估不同时间 CAR-T 表面耗竭分子的变化。我们发现利妥昔单抗组CAR-T细胞上耗竭分子(LAG-3、PD-1、TIM-3)的表达显着低于对照组。
结论
利妥昔单抗联合CAR-T治疗可有效改善多线治疗失败的B-ALL患者的长期预后。在体外,我们观察到利妥昔单抗可以通过使 ALL 对 CART 介导的细胞毒性敏感并减少 CAR-T 耗竭来提高 CAR-T 疗效。



























 京公网安备 11010802027423号
京公网安备 11010802027423号