Chemical Papers ( IF 2.2 ) Pub Date : 2024-04-24 , DOI: 10.1007/s11696-024-03441-2 Nameer Mazin Zeki , Yasser Fakri Mustafa
|
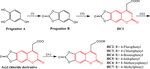
This study revealed the first synthesis of seven novel coumarins annulated with heterocycle composed of four heteroatoms, one sulfur, and three oxygen atoms in a linear pattern. This is an effort to find readily available coumarin frameworks with a broad variety of biological properties that are both adjustable and easy to get. The synthetic annulates' structural framework was confirmed by means of spectroscopic techniques, which included 1H-NMR, 13C-NMR, and FTIR. The synthesized annulates were investigated in vitro for their biomedical potential as antioxidative stress, anti-inflammatory, antidiabetic, anticancer, and antimicrobial agents. In addition, their biosafety toward nontumor cells and commensal bacterial strains was also assessed in vitro. Computer-aided programs were employed to explore the toxicity and pharmacokinetic profiles of the synthesized annulates. Based on the findings that were obtained, the authors stated the following main outcomes. There have been promising and far-reaching biological effects of the synthesized heterocyclic coumarin annulates. HC1 demonstrated strong anti-inflammatory potential through the lipoxygenase-dependent route. Moreover, HC1 exhibited significant antifungal efficacy, surpassing that of nystatin. HC2 held great promise as an antioxidative stress, anticancer, and biosafe candidate. HC3 exhibited a strong antibacterial potential against all tested aerobic bacterial strains, demonstrating a potency equivalent to that of ciprofloxacin. In addition, all of the synthesized annulates, especially HC3, exhibited a noteworthy biosafety profile against the commensal bacterial strains. The strong inhibitory capabilities of HC6 and HC7 toward glucosidase and amylase indicate that they possess great promise as antidiabetic agents. Finally, the synthesized annulates showed favorable toxicity and oral bioavailability properties. It can be inferred that these annulates have the potential to be useful frameworks for developing new drugs with a broad spectrum of bioactivity in the coming years.
中文翻译:

新型杂环香豆素环化物:合成及其在生物医学、实验室到临床研究中的作用
这项研究首次合成了七种新型香豆素,其杂环由四个杂原子、一个硫和三个线性氧原子组成。这是为了寻找现成的香豆素框架,其具有多种生物特性,既可调节又易于获得。合成环的结构框架通过光谱技术(包括1 H-NMR、13 C-NMR 和 FTIR)得到证实。在体外研究了合成的环状物作为抗氧化应激、抗炎、抗糖尿病、抗癌和抗菌剂的生物医学潜力。此外,还在体外评估了它们对非肿瘤细胞和共生细菌菌株的生物安全性。采用计算机辅助程序来探索合成环的毒性和药代动力学特征。根据所获得的发现,作者陈述了以下主要结果。合成的杂环香豆素环状化合物具有前景广阔且影响深远的生物学效应。HC1通过脂氧合酶依赖性途径表现出强大的抗炎潜力。此外,HC1表现出显着的抗真菌功效,超过制霉菌素。HC2作为抗氧化应激、抗癌和生物安全候选药物具有广阔的前景。HC3对所有测试的需氧菌株均表现出强大的抗菌潜力,其效力与环丙沙星相当。此外,所有合成的环状化合物,尤其是HC3,针对共生菌株表现出值得注意的生物安全性。HC6和HC7对葡萄糖苷酶和淀粉酶的强抑制能力表明它们作为抗糖尿病药物具有广阔的前景。最后,合成的环状化合物显示出良好的毒性和口服生物利用度特性。可以推断,这些环状结构有可能成为未来几年开发具有广泛生物活性的新药物的有用框架。



























 京公网安备 11010802027423号
京公网安备 11010802027423号