当前位置:
X-MOL 学术
›
Cancer Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Microwave ablation combined with PD‐L1 blockade synergistically promotes Cxcl9‐mediated antitumor immunity
Cancer Science ( IF 5.7 ) Pub Date : 2024-04-24 , DOI: 10.1111/cas.16182 Ningning He 1, 2, 3 , Hao Huang 2, 3 , Shaoxian Wu 2, 3 , Weipeng Ji 2, 3 , Yicheng Tai 2, 3 , Ruicheng Gao 2, 3 , Yingting Liu 2, 3 , Yungang Liu 2, 4 , Lujun Chen 2, 3 , Dawei Zhu 2, 3 , Xiao Zheng 2, 3 , Jingting Jiang 1, 2, 3
Cancer Science ( IF 5.7 ) Pub Date : 2024-04-24 , DOI: 10.1111/cas.16182 Ningning He 1, 2, 3 , Hao Huang 2, 3 , Shaoxian Wu 2, 3 , Weipeng Ji 2, 3 , Yicheng Tai 2, 3 , Ruicheng Gao 2, 3 , Yingting Liu 2, 3 , Yungang Liu 2, 4 , Lujun Chen 2, 3 , Dawei Zhu 2, 3 , Xiao Zheng 2, 3 , Jingting Jiang 1, 2, 3
Affiliation
|
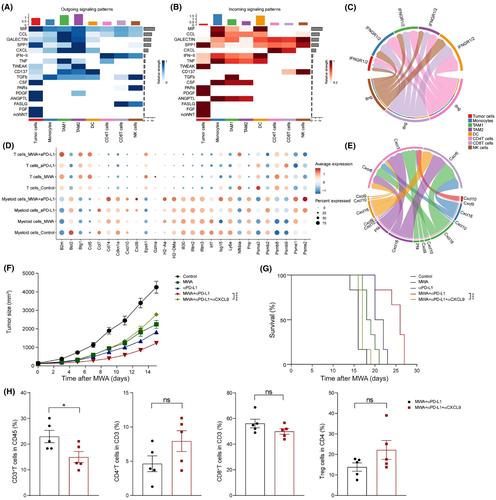
Although microwave ablation (MWA) is an important curative therapy in colorectal cancer liver metastasis, recurrence still occurs clinically. Our previous studies have shown that the expression of programmed cell death 1 ligand 1 (PD‐L1) is upregulated following MWA, suggesting that MWA combined with anti‐PD‐L1 treatment can serve as a promising clinical therapeutic strategy against cancer. Using MWA‐treated preclinical mice models, MWA combined with αPD‐L1 treatment decreased tumor growth and prolonged overall survival (OS). Furthermore, through flow cytometry and single‐cell RNA sequencing analysis, we determined that the MWA plus αPD‐L1 therapy significantly suppressed CD8+ T cell exhaustion and enhanced their effector function. A significant increase in γ‐interferon (IFN‐γ) stimulated transcription factors, specifically Irf8, was observed. This enhancement facilitated the polarization of tumor‐associated macrophages (TAM1s and TAM2s) through the nuclear factor‐κB/JAK‐STAT1 signaling pathway. Furthermore, the combination therapy stimulated the production of CXC motif chemokine ligand (CXCL9) by TAM1s and tumor cells, potentially increasing the chemotaxis of CD8 T cells and Th1 cells. Knocking out Cxcl9 in MC38 tumor cells or using CXCL9 blockade enhanced tumor growth of untreated tumors and shortened OS. Taken together, our study showed that blocking the IFN‐γ‐Cxcl9‐CD8+ T axis promoted tumor progression and discovered a potential involvement of IRF8‐regulated TAMs in preventing T cell exhaustion. Collectively, we identified that the combination of MWA with anti‐PD‐L1 treatment holds promise as a therapeutic strategy to rejuvenate the immune response against tumors. This merits further exploration in clinical studies.
中文翻译:

微波消融联合PD-L1阻断协同促进Cxcl9介导的抗肿瘤免疫
尽管微波消融(MWA)是结直肠癌肝转移的重要治疗方法,但临床上仍会出现复发情况。我们之前的研究表明,MWA 后程序性细胞死亡 1 配体 1 (PD-L1) 的表达上调,表明 MWA 联合抗 PD-L1 治疗可以作为一种有前景的临床癌症治疗策略。使用 MWA 治疗的临床前小鼠模型,MWA 联合 αPD-L1 治疗可减少肿瘤生长并延长总生存期 (OS)。此外,通过流式细胞术和单细胞 RNA 测序分析,我们确定 MWA 联合 αPD-L1 疗法显着抑制 CD8+ T 细胞耗竭并增强其效应功能。观察到 γ-干扰素 (IFN-γ) 刺激的转录因子,特别是 Irf8 显着增加。这种增强通过核因子-κB/JAK-STAT1信号通路促进了肿瘤相关巨噬细胞(TAM1和TAM2)的极化。此外,联合疗法刺激 TAM1 和肿瘤细胞产生 CXC 基序趋化因子配体 (CXCL9),可能增加 CD8 T 细胞和 Th1 细胞的趋化性。敲除 MC38 肿瘤细胞中的 Cxcl9 或使用 CXCL9 阻断可增强未经治疗肿瘤的肿瘤生长并缩短 OS。综上所述,我们的研究表明,阻断 IFN-γ-Cxcl9-CD8+ T 轴促进肿瘤进展,并发现 IRF8 调节的 TAM 可能参与防止 T 细胞耗竭。总的来说,我们发现 MWA 与抗 PD-L1 治疗的组合有望成为恢复肿瘤免疫反应的治疗策略。这值得临床研究进一步探索。
更新日期:2024-04-24
中文翻译:

微波消融联合PD-L1阻断协同促进Cxcl9介导的抗肿瘤免疫
尽管微波消融(MWA)是结直肠癌肝转移的重要治疗方法,但临床上仍会出现复发情况。我们之前的研究表明,MWA 后程序性细胞死亡 1 配体 1 (PD-L1) 的表达上调,表明 MWA 联合抗 PD-L1 治疗可以作为一种有前景的临床癌症治疗策略。使用 MWA 治疗的临床前小鼠模型,MWA 联合 αPD-L1 治疗可减少肿瘤生长并延长总生存期 (OS)。此外,通过流式细胞术和单细胞 RNA 测序分析,我们确定 MWA 联合 αPD-L1 疗法显着抑制 CD8



























 京公网安备 11010802027423号
京公网安备 11010802027423号