当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
The development of cage phosphine ‘DalPhos’ ligands to enable nickel-catalyzed cross-couplings of (hetero)aryl electrophiles
Chemical Science ( IF 8.4 ) Pub Date : 2024-04-25 , DOI: 10.1039/d4sc01253d Kathleen M. Morrison 1 , Mark Stradiotto 1
Chemical Science ( IF 8.4 ) Pub Date : 2024-04-25 , DOI: 10.1039/d4sc01253d Kathleen M. Morrison 1 , Mark Stradiotto 1
Affiliation
|
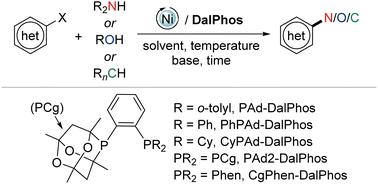
Nickel-catalyzed cross-couplings of (hetero)aryl electrophiles with a diversity of nucleophiles (nitrogen, oxygen, carbon, and others) have evolved into competitive alternatives to well-established palladium- and copper-based protocols for the synthesis of (hetero)aryl products, including (hetero)anilines and (hetero)aryl ethers. A survey of the literature reveals that the use of cage phosphine (CgP) ‘DalPhos’ (DALhousie PHOSphine) bisphosphine-type ligands operating under thermal conditions currently offers the most broad substrate scope in nickel-catalyzed cross-couplings of this type, especially involving (hetero)aryl chlorides and phenol-derived electrophiles. The development and application of these DalPhos ligands is described in a ligand-specific manner that is intended to serve as a guide for the synthetic chemistry end-user.
中文翻译:

笼状膦“DalPhos”配体的开发使镍催化的(杂)芳基亲电子试剂能够交叉偶联
(杂)芳基亲电子试剂与多种亲核试剂(氮、氧、碳等)的镍催化交叉偶联已发展成为用于合成(杂)的成熟的基于钯和铜的方案的竞争性替代方案芳基产品,包括(杂)苯胺和(杂)芳基醚。文献调查显示,在热条件下使用笼状膦 (CgP)“DalPhos”(DALhousie PHOSphine) 双膦型配体目前在此类镍催化交叉偶联中提供了最广泛的底物范围,特别是涉及(杂)芳基氯和苯酚衍生的亲电子试剂。这些 DalPhos 配体的开发和应用以配体特异性方式进行描述,旨在为合成化学最终用户提供指导。
更新日期:2024-04-29
中文翻译:

笼状膦“DalPhos”配体的开发使镍催化的(杂)芳基亲电子试剂能够交叉偶联
(杂)芳基亲电子试剂与多种亲核试剂(氮、氧、碳等)的镍催化交叉偶联已发展成为用于合成(杂)的成熟的基于钯和铜的方案的竞争性替代方案芳基产品,包括(杂)苯胺和(杂)芳基醚。文献调查显示,在热条件下使用笼状膦 (CgP)“DalPhos”(DALhousie PHOSphine) 双膦型配体目前在此类镍催化交叉偶联中提供了最广泛的底物范围,特别是涉及(杂)芳基氯和苯酚衍生的亲电子试剂。这些 DalPhos 配体的开发和应用以配体特异性方式进行描述,旨在为合成化学最终用户提供指导。



























 京公网安备 11010802027423号
京公网安备 11010802027423号