当前位置:
X-MOL 学术
›
J. Agric. Food Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Chemoenzymatic Asymmetric Synthesis of Chiral Triazole Fungicide (R)-Tebuconazole in High Optical Purity Mediated by an Epoxide Hydrolase from Rhodotorula paludigensis
Journal of Agricultural and Food Chemistry ( IF 6.1 ) Pub Date : 2024-04-25 , DOI: 10.1021/acs.jafc.3c07949 Die Hu 1 , Xue-Wei Jia 1 , Jia-Lan Lu 1 , Zhi-Yi Lu 1 , Cun-Duo Tang 2 , Feng Xue 3 , Chao Huang 4 , Qing-Gong Ren 5 , Yu-Cai He 1
Journal of Agricultural and Food Chemistry ( IF 6.1 ) Pub Date : 2024-04-25 , DOI: 10.1021/acs.jafc.3c07949 Die Hu 1 , Xue-Wei Jia 1 , Jia-Lan Lu 1 , Zhi-Yi Lu 1 , Cun-Duo Tang 2 , Feng Xue 3 , Chao Huang 4 , Qing-Gong Ren 5 , Yu-Cai He 1
Affiliation
|
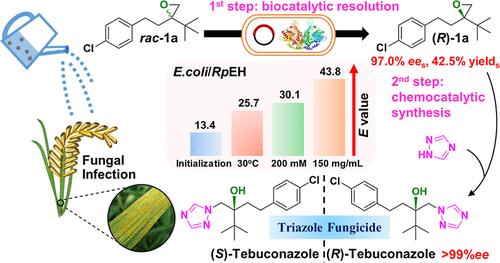
Tebuconazole is a chiral triazole fungicide used globally in agriculture as a racemic mixture, but its enantiomers exhibit significant enantioselective dissimilarities in bioactivity and environmental behaviors. The steric hindrance caused by the tert-butyl group makes it a great challenge to synthesize tebuconazole enantiomers. Here, we designed a simple chemoenzymatic approach for the asymmetric synthesis of (R)-tebuconazole, which includes the biocatalytic resolution of racemic epoxy-precursor (2-tert-butyl-2-[2-(4-chlorophenyl)ethyl] oxirane, rac-1a) by Escherichia coli/Rpeh whole cells expressed epoxide hydrolase from Rhodotorula paludigensis (RpEH), followed by a one-step chemocatalytic synthesis of (R)-tebuconazole. It was observed that (S)-1a was preferentially hydrolyzed by E. coli/Rpeh, whereas (R)-1a was retained with a specific activity of 103.8 U/g wet cells and a moderate enantiomeric ratio (E value) of 13.4, which was remarkably improved to 43.8 after optimizing the reaction conditions. Additionally, a gram-scale resolution of 200 mM rac-1a was performed using 150 mg/mL E. coli/Rpeh wet cells, resulting in the retention of (R)-1a in a 97.0% ees, a 42.5% yields, and a 40.5 g/L/d space-time yield. Subsequently, the synthesis of highly optical purity (R)-tebuconazole (>99% ee) was easily achieved through the chemocatalytic ring-opening of the epoxy-precursor (R)-1a with 1,2,4-triazole. To elucidate insight into the enantioselectivity, molecular docking simulations revealed that the unique L-shaped substrate-binding pocket of RpEH plays a crucial role in the enantioselective recognition of bulky 2,2-disubstituted oxirane 1a.
中文翻译:

由红酵母环氧化物水解酶介导的化学酶法不对称合成高光学纯度的手性三唑类杀菌剂 (R)-戊唑醇
戊唑醇是一种手性三唑类杀菌剂,作为外消旋混合物在全球农业中使用,但其对映体在生物活性和环境行为方面表现出显着的对映选择性差异。叔丁基造成的空间位阻给戊唑醇对映体的合成带来了巨大的挑战。在这里,我们设计了一种简单的化学酶法来不对称合成( R )-戊唑醇,其中包括外消旋环氧前体(2-叔丁基-2-[2-(4-氯苯基)乙基]环氧乙烷的生物催化拆分,rac - 1a)由大肠杆菌/ Rpeh全细胞表达来自Rhodotorula paludigensis的环氧化物水解酶(Rp EH),然后一步化学催化合成(R)-戊唑醇。观察到( S ) -1a优先被大肠杆菌/ Rpeh水解,而( R ) -1a被保留,比活性为103.8 U/g湿细胞,中等对映体比率(E值)为13.4,优化反应条件后,显着提高至43.8。此外,使用 150 mg/mL大肠杆菌/Rpeh湿细胞进行了200 mM rac - 1a的克级分辨率,结果 ( R )-1a的保留率为97.0% ee s ,产率 42.5% ,以及 40.5 g/L/d 的时空产率。随后,通过环氧前体( R ) -1a与1,2,4-三唑的化学催化开环,轻松合成了高光学纯度( R ) -戊唑醇(>99% ee )。为了阐明对映选择性,分子对接模拟表明, Rp EH独特的 L 形底物结合口袋在大体积 2,2-二取代环氧乙烷1a的对映选择性识别中发挥着至关重要的作用。
更新日期:2024-04-25
中文翻译:

由红酵母环氧化物水解酶介导的化学酶法不对称合成高光学纯度的手性三唑类杀菌剂 (R)-戊唑醇
戊唑醇是一种手性三唑类杀菌剂,作为外消旋混合物在全球农业中使用,但其对映体在生物活性和环境行为方面表现出显着的对映选择性差异。叔丁基造成的空间位阻给戊唑醇对映体的合成带来了巨大的挑战。在这里,我们设计了一种简单的化学酶法来不对称合成( R )-戊唑醇,其中包括外消旋环氧前体(2-叔丁基-2-[2-(4-氯苯基)乙基]环氧乙烷的生物催化拆分,rac - 1a)由大肠杆菌/ Rpeh全细胞表达来自Rhodotorula paludigensis的环氧化物水解酶(Rp EH),然后一步化学催化合成(R)-戊唑醇。观察到( S ) -1a优先被大肠杆菌/ Rpeh水解,而( R ) -1a被保留,比活性为103.8 U/g湿细胞,中等对映体比率(E值)为13.4,优化反应条件后,显着提高至43.8。此外,使用 150 mg/mL大肠杆菌/Rpeh湿细胞进行了200 mM rac - 1a的克级分辨率,结果 ( R )-1a的保留率为97.0% ee s ,产率 42.5% ,以及 40.5 g/L/d 的时空产率。随后,通过环氧前体( R ) -1a与1,2,4-三唑的化学催化开环,轻松合成了高光学纯度( R ) -戊唑醇(>99% ee )。为了阐明对映选择性,分子对接模拟表明, Rp EH独特的 L 形底物结合口袋在大体积 2,2-二取代环氧乙烷1a的对映选择性识别中发挥着至关重要的作用。



























 京公网安备 11010802027423号
京公网安备 11010802027423号