当前位置:
X-MOL 学术
›
Environ. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Unveiling the Function of Oxygen Vacancy on Facet-Dependent CeO2 for the Catalytic Destruction of Monochloromethane: Guidance for Industrial Catalyst Design
Environmental Science & Technology ( IF 11.4 ) Pub Date : 2024-04-26 , DOI: 10.1021/acs.est.4c00297 Yuetan Su 1 , Bowen Han 1 , Qingjie Meng 2 , Xueqing Luo 3 , Zhongbiao Wu 1, 4 , Xiaole Weng 1, 3
Environmental Science & Technology ( IF 11.4 ) Pub Date : 2024-04-26 , DOI: 10.1021/acs.est.4c00297 Yuetan Su 1 , Bowen Han 1 , Qingjie Meng 2 , Xueqing Luo 3 , Zhongbiao Wu 1, 4 , Xiaole Weng 1, 3
Affiliation
|
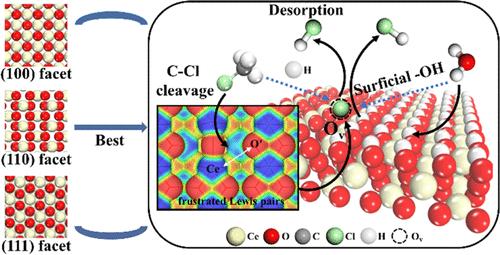
Secondary pollution remains a critical challenge for the catalytic destruction of chlorinated volatile organic compounds (CVOCs). By employing experimental studies and theoretical calculations, we provide valuable insights into the catalytic behaviors exhibited by ceria rods, cubes, and octahedra for monochloromethane (MCM) destruction, shedding light on the elementary reactions over facet-dependent CeO2. Our findings demonstrate that CeO2 nanorods with the (110) facet exhibit the best performance in MCM destruction, and the role of vacancies is mainly to form a longer distance (4.63 Å) of frustrated Lewis pairs (FLPs) compared to the stoichiometric surface, thereby enhancing the activation of MCM molecules. Subsequent molecular orbital analysis showed that the adsorption of MCM mainly transferred electrons from the 3σ and 4π* orbitals to the Ce 4f orbitals, and the activation was mainly caused by weakening of the 3σ bonding orbitals. Furthermore, isotopic experiments and theoretical calculations demonstrated that the hydrogen chloride generated is mainly derived from methyl in MCM rather than from water, and the primary function of water is to form excess saturated H on the surface, facilitating the desorption of generated hydrogen chloride.
中文翻译:

揭示面相关 CeO2 上氧空位对一氯甲烷催化破坏的作用:工业催化剂设计指南
二次污染仍然是催化破坏氯化挥发性有机化合物(CVOC)的关键挑战。通过实验研究和理论计算,我们对二氧化铈棒、立方体和八面体对一氯甲烷 (MCM) 破坏的催化行为提供了有价值的见解,揭示了晶面依赖性 CeO 2上的基元反应。我们的研究结果表明,具有(110)面的CeO 2纳米棒在MCM破坏方面表现出最佳性能,并且与化学计量表面相比,空位的作用主要是形成更长距离(4.63 Å)的受挫路易斯对(FLP),从而增强MCM分子的活化。随后的分子轨道分析表明,MCM的吸附主要将电子从3σ和4π * 轨道转移到Ce 4f轨道,而活化主要是由于3σ键合轨道的减弱引起的。此外,同位素实验和理论计算表明,产生的氯化氢主要来自MCM中的甲基而不是水,而水的主要作用是在表面形成过量的饱和H,促进产生的氯化氢的解吸。
更新日期:2024-04-26
中文翻译:

揭示面相关 CeO2 上氧空位对一氯甲烷催化破坏的作用:工业催化剂设计指南
二次污染仍然是催化破坏氯化挥发性有机化合物(CVOC)的关键挑战。通过实验研究和理论计算,我们对二氧化铈棒、立方体和八面体对一氯甲烷 (MCM) 破坏的催化行为提供了有价值的见解,揭示了晶面依赖性 CeO 2上的基元反应。我们的研究结果表明,具有(110)面的CeO 2纳米棒在MCM破坏方面表现出最佳性能,并且与化学计量表面相比,空位的作用主要是形成更长距离(4.63 Å)的受挫路易斯对(FLP),从而增强MCM分子的活化。随后的分子轨道分析表明,MCM的吸附主要将电子从3σ和4π * 轨道转移到Ce 4f轨道,而活化主要是由于3σ键合轨道的减弱引起的。此外,同位素实验和理论计算表明,产生的氯化氢主要来自MCM中的甲基而不是水,而水的主要作用是在表面形成过量的饱和H,促进产生的氯化氢的解吸。



























 京公网安备 11010802027423号
京公网安备 11010802027423号