当前位置:
X-MOL 学术
›
Mol. Biosyst.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Enantioselective recognition of an isomeric ligand by a biomolecule: mechanistic insights into static and dynamic enantiomeric behavior and structural flexibility
Molecular BioSystems Pub Date : 2017-08-18 00:00:00 , DOI: 10.1039/c7mb00378a Wei Peng 1, 2, 3, 4, 5 , Fei Ding 1, 2, 3, 4, 5
Molecular BioSystems Pub Date : 2017-08-18 00:00:00 , DOI: 10.1039/c7mb00378a Wei Peng 1, 2, 3, 4, 5 , Fei Ding 1, 2, 3, 4, 5
Affiliation
|
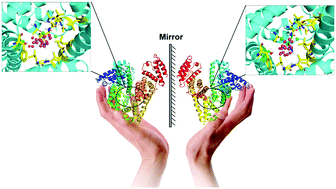
Chirality is a ubiquitous basic attribute of nature, which inseparably relates to the life activity of living organisms. However, enantiomeric differences have still failed to arouse enough attention during the biological evaluation and practical application of chiral substances, and this poses a large threat to human health. In the current study, we explore the enantioselective biorecognition of a chiral compound by an asymmetric biomolecule, and then decipher the molecular basis of such a biological phenomenon on the static and, in particular, the dynamic scale. In light of the wet experiments, in silico docking results revealed that the orientation of the latter part of the optical isomer structures in the recognition domain can be greatly affected by the chiral carbon center in a model ligand molecule, and this event may induce large disparities between the static chiral bioreaction modes and noncovalent interactions (especially hydrogen bonding). Dynamic stereoselective biorecognition assays indicated that the conformational stability of the protein–(S)-diclofop system is clearly greater than the protein–(R)-diclofop adduct; and moreover, the conformational alterations of the diclofop enantiomers in the dynamic process will directly influence the conformational flexibility of the key residues found in the biorecognition region. These points enable the changing trends of biopolymer structural flexibility and free energy to exhibit significant distinctions when proteins sterically recognize the (R)-/(S)-stereoisomers. The outcomes of the energy decomposition further showed that the van der Waals’ energy has roughly the same contribution to the chiral recognition biosystems, whereas the contribution of electrostatic energy to the protein–(R)-diclofop complex is notably smaller than to the protein–(S)-diclofop bioconjugate. This proves that differences in the noncovalent bonds would have a serious impact on the stereoselective biorecognition between a biomacromolecule and chiral ligand. The present scenario is expected to attract more interest from both researchers and administrative agencies, since in a chiral environment, enantioselectivity exists in all of the biochemical processes of a chiral chemical, and this might finally elicit the disparate biological activities of (R)-/(S)-enantiomers.
中文翻译:

生物分子对异构体配体的对映选择性识别:对静态和动态对映体行为及结构灵活性的机械见解
手性是自然界普遍存在的基本属性,它与生物体的生命活动密不可分。然而,在手性物质的生物学评估和实际应用中,对映异构体的差异仍然未能引起足够的重视,这对人类健康构成了巨大威胁。在当前的研究中,我们探索通过不对称生物分子对手性化合物的对映选择性生物识别,然后在静态范围内,特别是在动态范围内,破译这种生物现象的分子基础。根据湿实验,计算机模拟对接结果表明,识别配体域中光学异构体结构的后半部分的方向会受到模型配体分子中手性碳中心的极大影响,并且该事件可能会导致静态手性生物反应模式与非共价相互作用之间存在较大差异(尤其是氢键)。动态立体选择性生物识别分析表明,蛋白质-(S)-双链菌素系统的构象稳定性明显大于蛋白质-(R)-双氯芬加合物; 而且,在动态过程中,双氯芬普对映异构体的构象改变将直接影响生物识别区域中关键残基的构象柔性。当蛋白质在空间上识别(R)-/(S)-立体异构体时,这些观点使生物聚合物结构柔韧性和自由能的变化趋势表现出显着差异。能量分解的结果进一步表明,范德华斯能量对手性识别生物系统的贡献大致相同,而静电能量对蛋白质-(R)-二氟甲磺酸盐复合物的贡献明显小于对蛋白质- (S)-双氯芬酸生物共轭物。这证明了非共价键的差异将对生物大分子与手性配体之间的立体选择性生物识别产生严重影响。由于在手性环境中,手性化学物质的所有生化过程中都存在对映选择性,因此目前的情况预计将引起研究人员和行政机构的更多兴趣,这最终可能引发(R)-/的不同生物活性。(S)-对映异构体。
更新日期:2017-10-25
中文翻译:

生物分子对异构体配体的对映选择性识别:对静态和动态对映体行为及结构灵活性的机械见解
手性是自然界普遍存在的基本属性,它与生物体的生命活动密不可分。然而,在手性物质的生物学评估和实际应用中,对映异构体的差异仍然未能引起足够的重视,这对人类健康构成了巨大威胁。在当前的研究中,我们探索通过不对称生物分子对手性化合物的对映选择性生物识别,然后在静态范围内,特别是在动态范围内,破译这种生物现象的分子基础。根据湿实验,计算机模拟对接结果表明,识别配体域中光学异构体结构的后半部分的方向会受到模型配体分子中手性碳中心的极大影响,并且该事件可能会导致静态手性生物反应模式与非共价相互作用之间存在较大差异(尤其是氢键)。动态立体选择性生物识别分析表明,蛋白质-(S)-双链菌素系统的构象稳定性明显大于蛋白质-(R)-双氯芬加合物; 而且,在动态过程中,双氯芬普对映异构体的构象改变将直接影响生物识别区域中关键残基的构象柔性。当蛋白质在空间上识别(R)-/(S)-立体异构体时,这些观点使生物聚合物结构柔韧性和自由能的变化趋势表现出显着差异。能量分解的结果进一步表明,范德华斯能量对手性识别生物系统的贡献大致相同,而静电能量对蛋白质-(R)-二氟甲磺酸盐复合物的贡献明显小于对蛋白质- (S)-双氯芬酸生物共轭物。这证明了非共价键的差异将对生物大分子与手性配体之间的立体选择性生物识别产生严重影响。由于在手性环境中,手性化学物质的所有生化过程中都存在对映选择性,因此目前的情况预计将引起研究人员和行政机构的更多兴趣,这最终可能引发(R)-/的不同生物活性。(S)-对映异构体。



























 京公网安备 11010802027423号
京公网安备 11010802027423号