当前位置:
X-MOL 学术
›
Mol. Biosyst.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Real-time activity assays of β-lactamases in living bacterial cells: application to the inhibition of antibiotic-resistant E. coli strains
Molecular BioSystems Pub Date : 2017-09-04 00:00:00 , DOI: 10.1039/c7mb00487g Ying Ge 1, 2, 3, 4, 5 , Ya-Jun Zhou 1, 2, 3, 4, 5 , Ke-Wu Yang 1, 2, 3, 4, 5 , Yi-Lin Zhang 1, 2, 3, 4, 5 , Yang Xiang 1, 2, 3, 4, 5 , Yue-Juan Zhang 1, 2, 3, 4, 5
Molecular BioSystems Pub Date : 2017-09-04 00:00:00 , DOI: 10.1039/c7mb00487g Ying Ge 1, 2, 3, 4, 5 , Ya-Jun Zhou 1, 2, 3, 4, 5 , Ke-Wu Yang 1, 2, 3, 4, 5 , Yi-Lin Zhang 1, 2, 3, 4, 5 , Yang Xiang 1, 2, 3, 4, 5 , Yue-Juan Zhang 1, 2, 3, 4, 5
Affiliation
|
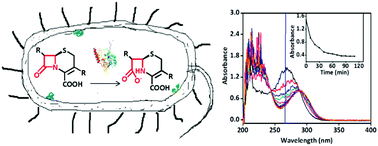
The emergence of antibiotic resistance caused by β-lactamases, including serine β-lactamases (SβLs) and metallo-β-lactamases (MβLs), is a global public health threat. L1, a B3 subclass MβL, hydrolyzes almost all of known β-lactam antibiotics. We report a simple and straightforward UV-Vis approach for real-time activity assays of β-lactamases inside living bacterial cells, and this method has been exemplified by choosing antibiotics, L1 enzyme, Escherichia coli expressing L1 (L1 E. coli), Escherichia coli expressing extended-spectrum β-lactamases (ESBL-E. coli), clinical bacterial strains, and reported MβL and SβL inhibitors. The cell-based studies demonstrated that cefazolin was hydrolyzed by L1 E. coli and clinical strains, and confirmed the hydrolysis to be inhibited by two known L1 inhibitors EDTA and azolylthioacetamide (ATAA), with an IC50 value of 1.6 and 18.9 μM, respectively. Also, it has been confirmed that the breakdown of cefazolin caused by ESBL-E. coli was inhibited by clavulanic acid, the first SβL inhibitor approved by FDA. The data gained through this approach are closely related to the biological function of the target enzyme in its physiological environment. The UV-Vis method proposed here can be applied to target-based whole-cell screening to search for potent β-lactamase inhibitors, and to assays of reactions in complex biological systems, for instance in medical assays.
中文翻译:

活细菌细胞中β-内酰胺酶的实时活性测定:在抑制抗生素抗性大肠杆菌菌株中的应用
由β-内酰胺酶(包括丝氨酸β-内酰胺酶(SβLs)和金属-β-内酰胺酶(MβLs))引起的抗生素耐药性的出现是全球性的公共卫生威胁。L1,是B3的MβL亚类,可水解几乎所有已知的β-内酰胺类抗生素。我们报告了一种简单直接的UV-Vis方法,用于活细菌细胞内的β-内酰胺酶的实时活性测定,并且该方法已通过选择抗生素,L1酶,表达L1的大肠杆菌(L1大肠杆菌),大肠杆菌得到了例证。大肠杆菌表达广谱β内酰胺酶(ESBL-大肠杆菌),临床细菌菌株,并报告MβL和SβL抑制剂。基于细胞的研究表明头孢唑啉被L1大肠杆菌水解和临床菌株,并确认水解被两种已知的L1抑制剂EDTA和偶氮基硫代乙酰胺(ATAA)抑制,其IC 50值分别为1.6和18.9μM 。另外,已经证实,由克拉维酸抑制了由ESBL-大肠杆菌引起的头孢唑啉的分解,克拉维酸是FDA批准的第一种SβL抑制剂。通过这种方法获得的数据与目标酶在其生理环境中的生物学功能密切相关。此处提出的UV-Vis方法可用于基于靶标的全细胞筛选,以寻找有效的β-内酰胺酶抑制剂,还可用于复杂的生物系统中的反应测定,例如医学测定。
更新日期:2017-10-25
中文翻译:

活细菌细胞中β-内酰胺酶的实时活性测定:在抑制抗生素抗性大肠杆菌菌株中的应用
由β-内酰胺酶(包括丝氨酸β-内酰胺酶(SβLs)和金属-β-内酰胺酶(MβLs))引起的抗生素耐药性的出现是全球性的公共卫生威胁。L1,是B3的MβL亚类,可水解几乎所有已知的β-内酰胺类抗生素。我们报告了一种简单直接的UV-Vis方法,用于活细菌细胞内的β-内酰胺酶的实时活性测定,并且该方法已通过选择抗生素,L1酶,表达L1的大肠杆菌(L1大肠杆菌),大肠杆菌得到了例证。大肠杆菌表达广谱β内酰胺酶(ESBL-大肠杆菌),临床细菌菌株,并报告MβL和SβL抑制剂。基于细胞的研究表明头孢唑啉被L1大肠杆菌水解和临床菌株,并确认水解被两种已知的L1抑制剂EDTA和偶氮基硫代乙酰胺(ATAA)抑制,其IC 50值分别为1.6和18.9μM 。另外,已经证实,由克拉维酸抑制了由ESBL-大肠杆菌引起的头孢唑啉的分解,克拉维酸是FDA批准的第一种SβL抑制剂。通过这种方法获得的数据与目标酶在其生理环境中的生物学功能密切相关。此处提出的UV-Vis方法可用于基于靶标的全细胞筛选,以寻找有效的β-内酰胺酶抑制剂,还可用于复杂的生物系统中的反应测定,例如医学测定。



























 京公网安备 11010802027423号
京公网安备 11010802027423号