当前位置:
X-MOL 学术
›
Mol. Biosyst.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Pre-steady-state kinetic analysis of damage recognition by human single-strand selective monofunctional uracil-DNA glycosylase SMUG1
Molecular BioSystems Pub Date : 2017-10-11 00:00:00 , DOI: 10.1039/c7mb00457e Alexandra A. Kuznetsova 1, 2, 3, 4 , Danila A. Iakovlev 1, 2, 3, 4 , Inna V. Misovets 3, 4, 5, 6, 7 , Alexander A. Ishchenko 8, 9, 10, 11, 12 , Murat K. Saparbaev 8, 9, 10, 11, 12 , Nikita A. Kuznetsov 1, 2, 3, 4, 5 , Olga S. Fedorova 1, 2, 3, 4, 5
Molecular BioSystems Pub Date : 2017-10-11 00:00:00 , DOI: 10.1039/c7mb00457e Alexandra A. Kuznetsova 1, 2, 3, 4 , Danila A. Iakovlev 1, 2, 3, 4 , Inna V. Misovets 3, 4, 5, 6, 7 , Alexander A. Ishchenko 8, 9, 10, 11, 12 , Murat K. Saparbaev 8, 9, 10, 11, 12 , Nikita A. Kuznetsov 1, 2, 3, 4, 5 , Olga S. Fedorova 1, 2, 3, 4, 5
Affiliation
|
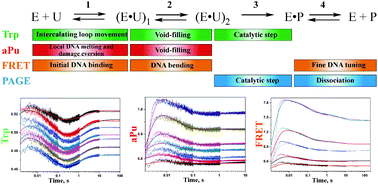
In all organisms, DNA glycosylases initiate base excision repair pathways resulting in removal of aberrant bases from DNA. Human SMUG1 belongs to the superfamily of uracil-DNA glycosylases catalyzing the hydrolysis of the N-glycosidic bond of uridine and uridine lesions bearing oxidized groups at C5: 5-hydroxymethyluridine (5hmU), 5-formyluridine (5fU), and 5-hydroxyuridine (5hoU). An apurinic/apyrimidinic (AP) site formed as the product of an N-glycosylase reaction is tightly bound to hSMUG1, thus inhibiting the downstream action of AP-endonuclease APE1. The steady-state kinetic parameters (kcat and KM; obtained from the literature) correspond to the enzyme turnover process limited by the release of hSMUG1 from the complex with the AP-site. In the present study, our objective was to carry out a stopped-flow fluorescence analysis of the interaction of hSMUG1 with a DNA substrate containing a dU:dG base pair to follow the pre-steady-state kinetics of conformational changes in both molecules. A comparison of kinetic data obtained by means of Trp and 2-aminopurine fluorescence and Förster resonance energy transfer (FRET) detection allowed us to elucidate the stages of specific and nonspecific DNA binding, to propose the mechanism of damaged base recognition by hSMUG1, and to determine the true rate of the catalytic step. Our results shed light on the kinetic mechanism underlying the initiation of base excision repair by hSMUG1 using the “wedge” strategy for DNA lesion search.
中文翻译:

人单链选择性单功能尿嘧啶-DNA糖基化酶SMUG1对损伤识别的稳态前动力学分析
在所有生物中,DNA糖基化酶均会启动碱基切除修复途径,从而从DNA中去除异常碱基。人SMUG1属于尿嘧啶DNA糖基化酶超家族,催化尿苷和尿苷病变的N-糖苷键水解,并在C5处带有氧化基团的尿苷病变:5-羟甲基尿苷(5hmU),5-甲酰基尿苷(5fU)和5-羟基尿苷5hoU)。作为N-糖基化酶反应产物而形成的嘌呤/嘧啶(AP)位点与hSMUG1紧密结合,从而抑制了AP-核酸内切酶APE1的下游作用。稳态动力学参数(k cat和K M; (从文献中获得)对应于酶更新过程,该过程受具有AP位点的复合物中hSMUG1的释放所限制。在本研究中,我们的目标是对hSMUG1与包含dU:dG碱基对的DNA底物的相互作用进行停流荧光分析,以追踪两个分子构象变化的稳态前动力学。通过Trp和2-氨基嘌呤荧光以及Förster共振能量转移(FRET)检测获得的动力学数据的比较,使我们能够阐明特异性和非特异性DNA结合的阶段,提出hSMUG1识别碱基的机制,并确定催化步骤的真实速率。
更新日期:2017-11-21
中文翻译:

人单链选择性单功能尿嘧啶-DNA糖基化酶SMUG1对损伤识别的稳态前动力学分析
在所有生物中,DNA糖基化酶均会启动碱基切除修复途径,从而从DNA中去除异常碱基。人SMUG1属于尿嘧啶DNA糖基化酶超家族,催化尿苷和尿苷病变的N-糖苷键水解,并在C5处带有氧化基团的尿苷病变:5-羟甲基尿苷(5hmU),5-甲酰基尿苷(5fU)和5-羟基尿苷5hoU)。作为N-糖基化酶反应产物而形成的嘌呤/嘧啶(AP)位点与hSMUG1紧密结合,从而抑制了AP-核酸内切酶APE1的下游作用。稳态动力学参数(k cat和K M; (从文献中获得)对应于酶更新过程,该过程受具有AP位点的复合物中hSMUG1的释放所限制。在本研究中,我们的目标是对hSMUG1与包含dU:dG碱基对的DNA底物的相互作用进行停流荧光分析,以追踪两个分子构象变化的稳态前动力学。通过Trp和2-氨基嘌呤荧光以及Förster共振能量转移(FRET)检测获得的动力学数据的比较,使我们能够阐明特异性和非特异性DNA结合的阶段,提出hSMUG1识别碱基的机制,并确定催化步骤的真实速率。



























 京公网安备 11010802027423号
京公网安备 11010802027423号