Journal of Catalysis ( IF 6.5 ) Pub Date : 2019-07-10 , DOI: 10.1016/j.jcat.2019.06.037
Xue Zhang , Yong Li , Pu Guo , Jia-Bo Le , Zhi-You Zhou , Jun Cheng , Shi-Gang Sun
|
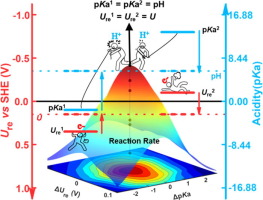
Understanding the effects of pH and potential on proton-coupled electron transfer (PCET) steps and optimizing the reaction activity are of key importance for efficient applications of energy conversion processes. In this work, we develop a simple theory to optimize the electrocatalytic PCET reactions, in which electron and proton transfer take place in sequential steps, by combining an energy level diagram and micro-kinetic theories. Our theory suggests matching conditions on PCET energetics (redox potentials and pKa’s) and reaction environments (potential and pH) in order to achieve maximum kinetics. Consequently, a descriptor representing deviation from the ideal condition, i.e. |ΔUre|+ 2.30kBT/e |ΔpKa|, is proposed to measure catalyst (in)activity. To test our theory, we further investigate CO2 electroreduction on a model catalyst both computationally and experimentally, and show that the activity for CO2 reduction on FePc (Iron Phthalocyanine) molecule is higher than H2 evolution at near neutral condition. Our theory not only identifies the individual contribution of electron and proton transfer to overpotentials but also provides simple guidelines for optimizing the experimental conditions and searching for efficient catalysts.
中文翻译:

优化电催化质子偶联电子转移反应活性的理论
理解pH和电势对质子偶联电子转移(PCET)步骤的影响并优化反应活性对于能量转化过程的有效应用至关重要。在这项工作中,我们通过结合能级图和微动力学理论,开发了一种简单的理论来优化电催化PCET反应,在该过程中,电子和质子转移按顺序进行。我们的理论建议在PCET能量学(氧化还原电势和p K a ′s)和反应环境(电势和pH)上匹配条件,以实现最大的动力学。因此,表示从理想状态,即偏差的描述符|Δ Ü重新| + 2.30 ķ乙T / ë|δP ķ一个|,提出了以测量催化剂(在)活性。为了验证我们的理论,我们在计算和实验上进一步研究了在模型催化剂上进行的CO 2电还原,并表明在接近中性条件下,FePc(酞菁铁)分子的CO 2还原活性高于H 2析出。我们的理论不仅确定了电子和质子转移对超电势的单独贡献,而且为优化实验条件和寻找有效的催化剂提供了简单的指南。





























































 京公网安备 11010802027423号
京公网安备 11010802027423号