当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Chain propagation determines the chemo- and regioselectivity of alkyl radical additions to C–O vs. C–C double bonds
Chemical Science ( IF 8.4 ) Pub Date : 2019-11-26 , DOI: 10.1039/c9sc04846d Tiffany O. Paulisch 1, 2, 3, 4 , Felix Strieth-Kalthoff 1, 2, 3, 4 , Christian Henkel 4, 5, 6, 7 , Lena Pitzer 1, 2, 3, 4 , Dirk M. Guldi 4, 5, 6, 7 , Frank Glorius 1, 2, 3, 4
Chemical Science ( IF 8.4 ) Pub Date : 2019-11-26 , DOI: 10.1039/c9sc04846d Tiffany O. Paulisch 1, 2, 3, 4 , Felix Strieth-Kalthoff 1, 2, 3, 4 , Christian Henkel 4, 5, 6, 7 , Lena Pitzer 1, 2, 3, 4 , Dirk M. Guldi 4, 5, 6, 7 , Frank Glorius 1, 2, 3, 4
Affiliation
|
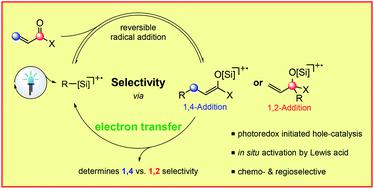
Investigations into the selectivity of intermolecular alkyl radical additions to C–O- vs. C–C-double bonds in α,β-unsaturated carbonyl compounds are described. Therefore, a photoredox-initiated radical chain reaction is explored, where the activation of the carbonyl-group through an in situ generated Lewis acid – originating from the substrate – enables the formation of either C–O or the C–C-addition products. α,β-Unsaturated aldehydes form selectively 1,2-, while esters and ketones form the corresponding 1,4-addition products exclusively. Computational studies lead to reason that this chemo- and regioselectivity is determined by the consecutive step, i.e. an electron transfer, after reversible radical addition, which eventually propagates the radical chain.
中文翻译:

链的传播决定了烷基对C–O和CC–C双键的化学选择性和区域选择性
描述了对α,β-不饱和羰基化合物中的C-O-与CC-双键的分子间烷基加成的选择性的研究。因此,探索了光氧化还原引发的自由基链反应,其中通过原位产生的路易斯酸(源自底物)活化羰基基团,可以形成C–O或C–C加成产物。α,β-不饱和醛选择性地形成1,2-,而酯和酮仅形成相应的1,4-加成产物。计算研究导致这种化学和区域选择性是由连续步骤确定的,即在可逆自由基添加后电子转移,该电子转移最终会扩散自由基链。
更新日期:2019-11-26
中文翻译:

链的传播决定了烷基对C–O和CC–C双键的化学选择性和区域选择性
描述了对α,β-不饱和羰基化合物中的C-O-与CC-双键的分子间烷基加成的选择性的研究。因此,探索了光氧化还原引发的自由基链反应,其中通过原位产生的路易斯酸(源自底物)活化羰基基团,可以形成C–O或C–C加成产物。α,β-不饱和醛选择性地形成1,2-,而酯和酮仅形成相应的1,4-加成产物。计算研究导致这种化学和区域选择性是由连续步骤确定的,即在可逆自由基添加后电子转移,该电子转移最终会扩散自由基链。



























 京公网安备 11010802027423号
京公网安备 11010802027423号