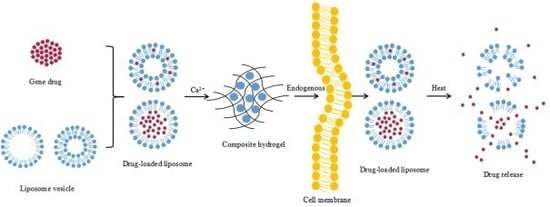
Design, Synthesis and Characterization of a Novel Type of Thermo-Responsible Phospholipid Microcapsule–Alginate Composite Hydrogel for Drug Delivery
Abstract
:1. Introduction
2. Results and Discussion
2.1. Preparation of and Characterization of the TSPMAH
2.1.1. Preparation of Thermosensitive Phospholipid Microcapsules
2.1.2. SEM and FTIR Characterization of the TSPMAH
2.1.3. Rheological Behavior of the TSPMAH
2.1.4. Analysis of Swelling Degree of TSPMAH
2.2. Analysis of Drug Loading Effect of TSPMAH
2.3. Analysis of Drug Release of the TSPMAH
2.4. Cytotoxicity Analysis of TSPMAH
3. Materials and Methods
3.1. Chemicals and Materials
3.2. Preparation of TSPMAH
3.3. Measurements of the Zeta Potentials of Phospholipid Microcapsules
3.4. FTIR Characterizations of the TSPMAH
3.5. SEM and TEM Observations of TSPMAH
3.6. DSC Analysis of the Thermo-Responsibilities of the Phospholipid Microcapsules
3.7. Sizing of the Thermo-Responsible Phospholipid Microcapsules
3.8. The Rheological Properties of the TSPMAH
3.9. Drug Loading and Drug Release of TSPMAH
3.10. Determination of the Swelling Ratio of the Composite Hydrogel
3.11. Cytotoxicity Study of TSPMAH
3.12. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Mulvey, M.A.; Hultgren, S.J. Cell biology. Bacterial spelunkers. Science 2000, 289, 732–733. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Ling, J.; Li, M.H. Physical stimuli-responsive liposomes and polymersomes as drug delivery vehicles based on phase transitions in the membrane. Nanoscale 2018, 10, 6781–6800. [Google Scholar] [CrossRef] [PubMed]
- Abu Lila, A.S.; Ishida, T. Liposomal delivery systems: design optimization and current applications. Biol. Pharm. Bull. 2017, 40, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kneidl, B.; Peller, M.; Winter, G.; Lindner, L.H.; Hossann, M. Thermosensitive liposomal drug delivery systems: state of the art review. Int. J. Nanomed. 2014, 9, 4387–4398. [Google Scholar] [CrossRef] [Green Version]
- May, J.P.; Ernsting, M.J.; Undzys, E.; Li, S.D. Thermosensitive liposomes for the delivery of gemcitabine and oxaliplatin to tumors. Mol. Pharmaceut. 2013, 10, 4499–4508. [Google Scholar] [CrossRef]
- Dicheva, B.M.; ten Hagen, T.L.; Schipper, D.; Seynhaeve, A.L.; van Rhoon, G.C.; Eggermont, A.M.M.; Koning, G.A. Targeted and heat-triggered doxorubicin delivery to tumors by dual targeted cationic thermosensitive liposomes. J. Control. Release 2014, 195, 37–48. [Google Scholar] [CrossRef]
- Peng, S.; Zou, L.; Liu, W.; Li, Z.; Liu, W.; Hu, X.; Chen, X.; Liu, C. Hybrid liposomes composed of amphiphilic chitosan and phospholipid: preparation, stability and bioavailability as a carrier for curcumin. Carbohyd. Polym. 2017, 156, 322–332. [Google Scholar] [CrossRef]
- Chen, J.; He, C.Q.; Lin, A.H.; Gu, W.; Chen, Z.P.; Li, W.; Cai, B.C. Thermosensitive liposomes with higher phase transition temperature for targeted drug delivery to tumor. Int. J. Pharmaceut. 2014, 475, 408–415. [Google Scholar] [CrossRef]
- Piffoux, M.; Silva, A.K.A.; Wilhelm, C.; Gazeau, F.; Tareste, D. Modification of extracellular vesicles by fusion with liposomes for the design of personalizaed biogenic drug delivery systems. ACS Nano 2018, 12, 6830–6842. [Google Scholar] [CrossRef]
- Al-Ahmady, Z.; Lozano, N.; Mei, K.C.; Al-Jamal, W.T.; Kostarelos, K. Engineering thermosensitive liposome-nanoparticle hybrids loaded with doxorubicin for heat-triggered drug release. Int. J. Pharmaceut. 2016, 514, 133–141. [Google Scholar] [CrossRef] [Green Version]
- Xu, X.; Xiao, X.; Wang, Y.; Xu, S.; Liu, H. Modulation of phase transition of thermosensitive liposomes by leucine zipper-structured lipopeptides. Phys. Chem. Chem. Phys. 2018, 20, 15916–15925. [Google Scholar] [CrossRef] [PubMed]
- Ta, T.; Convertine, A.J.; Reyes, C.R.; Stayton, P.S.; Porter, T.M. Thermosensitive liposomes modified with poly (N-isopropylacrylamide-co-propylacrylic acid) copolymers for triggered release of doxorubicin. Biomacromolecules 2010, 11, 1915–1920. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hashemi, M.; Omidi, M.; Muralidharan, B.; Tayebi, L.; Herpin, M.J.; Mohagheghi, M.A.; Mohammadi, J.; Smyth, H.D.C.; Milner, T.E. Layer-by-layer assembly of graphene oxide on thermosensitive liposomes for photo-chemotherapy. Acta Biomater. 2017, 65, 376–392. [Google Scholar] [CrossRef]
- Zhou, S.; Zhang, T.; Peng, B.; Luo, X.; Liu, X.; Hu, L.; Liu, Y.; Di, D.; Song, Y.; Deng, Y. Targeted delivery of epirubicin to tumor-associated macrophages by sialic acid-cholesterol conjugate modified liposomes with improved antitumor activity. Int. J. Pharmaceut. 2017, 523, 203–216. [Google Scholar] [CrossRef] [PubMed]
- Tagami, T.; Ernsting, M.J.; Li, S.D. Optimization of a novel and improved thermosensitive liposome formulated with DPPC and a Brij surfactant using a robust in vitro system. J. Control. Release 2011, 154, 290–297. [Google Scholar] [CrossRef]
- Tagami, T.; May, J.P.; Ernsting, M.J.; Li, S.D. A thermosensitive liposome prepared with a Cu2+ gradient demonstrates improved pharmacokinetics, drug delivery and antitumor efficacy. J. Control. Release 2012, 161, 142–149. [Google Scholar] [CrossRef]
- Lee, K.Y.; Mooney, D.J. Alginate: properties and biomedical applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef] [Green Version]
- Tran, N.M.; Dufresne, M.; Helle, F.; Hoffmann, T.W.; François, C.; Brochot, E.; Paullier, P.; Legallais, C.; Duverlie, G.; Castelain, S. Alginate hydrogel protects encapsulated hepatic HuH-7 cells against hepatitis C virus and other viral infections. PloS ONE 2014, 9, e109969. [Google Scholar] [CrossRef]
- Rassu, G.; Salis, A.; Porcu, E.P.; Giunchedi, P.; Roldo, M.; Gavini, E. Composite chitosan/alginate hydrogel for controlled release of deferoxamine: A system to potentially treat iron dysregulation diseases. Carbohyd. Polym. 2016, 136, 1338–1347. [Google Scholar] [CrossRef] [Green Version]
- Harper, B.A.; Barbut, S.; Lim, L.T.; Marcone, M.F. Effect of various gelling cations on the physical properties of “wet” alginate films. J. Food Sci. 2014, 79, E562–E567. [Google Scholar] [CrossRef]
- Gombotz, W.R.; Wee, S.F. Protein release from alginate matrices. Adv. Drug Deliv. Rev. 2012, 64, 194–205. [Google Scholar] [CrossRef]
- Treenate, P.; Monvisade, P. In vitro, drug release profiles of pH-sensitive hydroxyethylacryl chitosan/sodium alginate hydrogels using paracetamol as a soluble model drug. Int. J. Biol. Macromol. 2017, 99, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; Shi, M.; Zhang, W.; Shen, S.; Yue, Z.; Yang, H.; Ding, L.; Pan, X. Preparation, characterization and toxicological analysis of alginate-phospholipid microcapsule composite hydrogel. Chem. J. Chinese U. 2017, 38, 1270–1277. [Google Scholar] [CrossRef]
- Segawa, K.; Nagata, S. An apoptotic ‘Eat Me’ signal: phosphatidylserine exposure. Trends Cell Biol. 2015, 25, 639–650. [Google Scholar] [CrossRef] [PubMed]
- Fadok, V.A.; Bratton, D.L.; Frasch, S.C.; Warner, M.L.; Henson, P.M. The role of phosphatidylserine in recognition of apoptotic cells by phagocytes. Cell Death Differ. 1998, 5, 551–562. [Google Scholar] [CrossRef] [Green Version]
- Sabın, J.; Prieto, G.; Ruso, J.M.; Sarmiento, F. Fractal aggregates induced by liposome-liposome interaction in the presence of Ca2+. Eur. Phys. J. E 2007, 24, 201–210. [Google Scholar] [CrossRef]
- Mady, M.M.; Elshemey, W.M. Interaction of dipalmitoyl phosphatidylcholine (DPPC) liposomes and insulin. Mol. Phys. 2011, 109, 1593–1598. [Google Scholar] [CrossRef]
- Li, Z.; Peng, S.; Chen, X.; Zhu, Y.; Zou, L.; Liu, W.; Liu, C. Pluronics modified liposomes for curcumin encapsulation: Sustained release, stability and bioaccessibility. Food Res. Int. 2018, 108, 246–253. [Google Scholar] [CrossRef]
- Pérez-Madrigal, M.M.; Torras, J.; Casanovas, J.; Häring, M.; Aleman, C.; Díaz, D.D. A paradigm shifts for preparing versatile M2+-free gels from unmodified sodium alginate. Biomacromolecule. 2017, 18, 2967–2979. [Google Scholar] [CrossRef]
- Piai, J.F.; de Moura, M.R.; Rubira, A.F.; Muniz, E.C. Kinetic study of bovine serum albumin (BSA) released from alginate-Ca2+/PNIPAAm hydrogels. Macromol. Symp. 2010, 266, 108–113. [Google Scholar] [CrossRef]
- Chen, M.; Wang, L.; Chung, J.; Kim, Y.H.; Atluri, P.; Burdick, J.A. Methods to assess shear-thinning hydrogels for application as injectable biomaterials. ACS Biomater-Sci. Eng. 2017, 3, 3146–3160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nizam El-Din, H.M.; Abou Taleb, M.F.; El-Naggar, A.W.M. Metal sorption and swelling characters of acrylic acid and sodium alginate-based hydrogels synthesized by gamma irradiation. Nucl. Instrum. Meth. B 2018, 266, 2607–2613. [Google Scholar] [CrossRef]
- Shmeeda, H.; Amitay, Y.; Gorin, J.; Tzemach, D.; Mak, L.; Stern, S.T.; Barenholz, Y.; Gabizon, A. Co-encapsulation of alendronate and doxorubicin in pegylated liposomes: a novel formulation for chemo-immunotherapy of cancer. J. Drug Target. 2016, 24, 878–889. [Google Scholar] [CrossRef] [PubMed]
- Christian, D.A.; Cai, S.; Bowen, D.M.; Kim, Y.; Pajerowski, J.D.; Discher, D.E. Polymersome carriers: From self-assembly to siRNA and protein therapeutics. Eur. J. Pharm. Bio. 2009, 71, 463–474. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Immordino, M.L.; Brusa, P.; Arpicco, S.; Stella, B.; Dosio, F.; Cattel, L. Preparation, characterization, cytotoxicity and pharmacokinetics of liposomes containing docetaxel. J. Control. Release 2003, 91, 417–429. [Google Scholar] [CrossRef]
- Pupo, E.; Padrón, A.; Santana, E.; Sotolongo, J.; Quintana, D.; Dueñas, S.; Duarte, C.; De La, R.; Maria, C.; Hardy, E. Preparation of plasmid DNA-containing liposomes using a high-pressure homogenization-extrusion technique. J. Control. Release 2005, 104, 379–396. [Google Scholar] [CrossRef]
- Ho, L.; Bokharaei, M.; Li, S.D. Current update of a thermosensitive liposomes composed of DPPC and Brij78. J. Drug Target. 2018, 26, 1–13. [Google Scholar] [CrossRef]
- Rashidzadeh, A.; Olad, A.; Salari, D.; Reyhanitabar, A. On the preparation and swelling properties of hydrogel nanocomposite based on Sodium alginate-g-Poly (acrylic acid-co-acrylamide)/Clinoptilolite and its application as slow release fertilizer. J. Polym. Res. 2014, 21, 344. [Google Scholar] [CrossRef]
- Shi, M.; Jiang, R.; Cui, X.; Zhang, X.; Shen, S.; Ding, L.; Pan, X. Preparation, structure and pharmacodynamic analysis of protamine-siRNA complexes with different morphologies. Chem. J. Chinese U. 2019, 40, 1164–1171. [Google Scholar] [CrossRef]
- Chang, M.; Lu, S.; Zhang, F.; Zuo, T.; Guan, Y.; Wei, T.; Shao, W.; Lin, G. RGD-modified pH-sensitive liposomes for docetaxel tumor targeting. Colloid. Surface. B. 2015, 129, 175–182. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds TSPMAH, protamine–siRNA complex are available from the authors. |








| [Ca2+] (mM) | 0 | 10 | 20 | 30 | 40 |
|---|---|---|---|---|---|
| D (90%) (d. nm) | 224.6 ± 3.2 | 221.5 ± 1.9 | 226.2 ± 4.3 | 223.7 ± 2.5 | 220.3 ± 2.7 |
| Average Size (d. nm) | 124.3 ± 1.1 | 125.5 ± 2.4 | 126.2 ± 3.7 | 133.8 ± 2.8 | 127.6 ± 3.0 |
| Temperature (°C) | 25 | 37 | 40 | 43 |
|---|---|---|---|---|
| Protamine–siRNA complexes releasing rates (%) | 26.32 | 45.08 | 82.56 | 92.15 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ding, L.; Cui, X.; Jiang, R.; Zhou, K.; Wen, Y.; Wang, C.; Yue, Z.; Shen, S.; Pan, X. Design, Synthesis and Characterization of a Novel Type of Thermo-Responsible Phospholipid Microcapsule–Alginate Composite Hydrogel for Drug Delivery. Molecules 2020, 25, 694. https://doi.org/10.3390/molecules25030694
Ding L, Cui X, Jiang R, Zhou K, Wen Y, Wang C, Yue Z, Shen S, Pan X. Design, Synthesis and Characterization of a Novel Type of Thermo-Responsible Phospholipid Microcapsule–Alginate Composite Hydrogel for Drug Delivery. Molecules. 2020; 25(3):694. https://doi.org/10.3390/molecules25030694
Chicago/Turabian StyleDing, Liang, Xinxia Cui, Rui Jiang, Keya Zhou, Yalei Wen, Chenfeng Wang, Zhilian Yue, Shigang Shen, and Xuefeng Pan. 2020. "Design, Synthesis and Characterization of a Novel Type of Thermo-Responsible Phospholipid Microcapsule–Alginate Composite Hydrogel for Drug Delivery" Molecules 25, no. 3: 694. https://doi.org/10.3390/molecules25030694