Abstract
Mechanisms underlying the SARS-CoV-2-triggered hyperacute thrombo-inflammatory response that causes multi-organ damage in coronavirus disease 2019 (COVID-19) are poorly understood. Several lines of evidence implicate overactivation of complement. To delineate the involvement of complement in COVID-19, we prospectively studied 25 ICU-hospitalized patients for up to 21 days. Complement biomarkers in patient sera and healthy controls were quantified by enzyme-linked immunosorbent assays. Correlations with respiratory function and mortality were analyzed. Activation of complement via the classical/lectin pathways was variably increased. Strikingly, all patients had increased activation of the alternative pathway (AP) with elevated levels of activation fragments, Ba and Bb. This was associated with a reduction of the AP negative regulator, factor (F) H. Correspondingly, terminal pathway biomarkers of complement activation, C5a and sC5b-9, were significantly elevated in all COVID-19 patient sera. C5a and AP constituents Ba and Bb, were significantly associated with hypoxemia. Ba and FD at the time of ICU admission were strong independent predictors of mortality in the following 30 days. Levels of all complement activation markers were sustained throughout the patients’ ICU stays, contrasting with the varying serum levels of IL-6, C-reactive protein, and ferritin. Severely ill COVID-19 patients have increased and persistent activation of complement, mediated strongly via the AP. Complement activation biomarkers may be valuable measures of severity of lung disease and the risk of mortality. Large-scale studies will reveal the relevance of these findings to thrombo-inflammation in acute and post-acute COVID-19.
Similar content being viewed by others
Introduction
Despite vaccines targeting SARS-CoV-2, COVID-19 is likely to remain a major health and socioeconomic burden. The emergence of variant forms of the virus with increased virulence and/or transmissibility, raises concerns that SARS-CoV-2 may not be eradicated. Indeed, some predict that the pandemic will transition to endemic, causing multiple reinfections [1]. It is furthermore widely recognized that there are long-term and often serious sequelae of SARS-CoV-2 infections, and these will pose further challenges to healthcare systems and economies worldwide [2]. It is clear that there remains an urgent need to increase our understanding of the pathogenesis of COVID-19, in the hopes that such knowledge will yield better diagnostic and therapeutic strategies.
Although the clinical course varies, COVID-19 may manifest as a rapidly progressive, multi-organ thrombo-inflammatory disorder—a clinico-pathologic presentation consistent with an innate immune host response that has escalated out of control. Several lines of evidence point to overactivation of the blood-borne complement system, a major component of innate immunity, as playing a key role, not only in the inflammatory syndrome, but also in the micro- and macro-vascular thrombosis that commonly occurs [3,4,5,6,7,8,9,10,11].
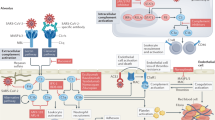
The complement system comprises > 40 soluble and membrane-bound proteins, serving to rapidly recognize and discard foreign pathogens and damaged host cells, recruit inflammatory cells, promote adaptive immunity, and facilitate healing (reviewed [12, 13]). Complement can be activated via the classical (CP), lectin (LP), and alternative (AP) pathways. The CP is initiated primarily on immune complexes via recognition of IgG, IgM or pentraxins by C1q, a component of the C1 complex. This sequentially activates C1r and C1s, leading to proteolysis of complement factors C4 and C2 to generate the CP C3 convertase, C4b2b, and liberation of activation fragments C4a and C2a. The LP is initiated by binding of complexes of mannose-binding lectin (MBL), collectins or ficolins and MBL-associated serine proteases (MASPs) to microbial pathogen surfaces. Similar to C1s, MASP-2 cleaves C4 and C2 to generate the LP, C3 convertase C4b2b. The AP is constitutively active at a low level by continuous hydrolysis of C3 to a C3b-like moiety, C3(H2O). Circulating factor D (FD) cleaves factor B, liberating activation fragment Ba and generating Bb complexed to C3b, thus forming the AP C3 convertase, C3bBb. The C3 convertases cleave C3–C3b, promoting feedback amplification of the cascade that generates more C3 convertase and addition of C3b molecules to the AP and CP/LP C3 convertases to form the respective C5 convertases. Cleavage of C5 releases the potent anaphylatoxin C5a, while C5b spontaneously assembles with C6, C7, C8 and multiple C9 molecules to form the lytic C5b-9 membrane attack complex (MAC), which also circulates in a soluble form (sC5b-9) [14]. Several soluble and membrane-anchored proteins, including serum factor H (FH), prevent excess complement activation. Diminished functional expression of one or more of these negative regulators, increases the risk of atypical hemolytic uremic syndrome (aHUS) with diffuse microvascular thrombosis, inflammation and multi-organ damage, a syndrome with features similar to those of COVID-19 [15,16,17,18,19,20,21,22,23]. Notably, anti-complement interventions are highly effective in preventing thrombosis in aHUS [24].
To gain further insights into the role of complement activation in COVID-19, we prospectively evaluated 25 critically ill patients with COVID-19 over the course of their stay in the intensive care unit (ICU), correlating serum levels of complement activation markers with clinical data and pulmonary outcomes (e.g., PaO2/FIO2 and static lung compliance), comparing the findings with those from a healthy cohort. We show that complement activation via all three pathways, but most prominently the AP, is markedly elevated in all critically ill COVID-19 patients; that this is sustained for the duration of their ICU stay up to 21 days; and that specific markers of complement activation correlate with lung function and are strong independent predictors of mortality. Our findings reveal potentially important biomarkers of disease activity and severity that may be useful in the design of treatments for acute and chronic COVID-19.
Materials and methods
Human ethics and biosafety
This study was conducted in accordance with the most current Declaration of Helsinki and with approval of the University of British Columbia Clinical Research Ethics Board (H20-00971). Informed written consent was obtained from all participants. Samples of blood from COVID-19 patients were obtained from an existing arterial line and handled in accordance with safety protocols approved by the University of British Columbia Biosafety Committee (B17-0174).
Study site, recruitment, and sample composition
Participants were 25 critically ill COVID-19 patients admitted to the Intensive Care Unit (ICU) of Vancouver General Hospital between March 24, 2020 and May 9, 2020 following a diagnosis of pneumonia associated with SARS-CoV-2 infection. Diagnoses were confirmed by reverse transcription polymerase chain reaction-positive nasopharyngeal or tracheal swabs. Details of the management of these patients have been previously reported [25]. Patients were anonymized and assigned enrolment numbers sequentially. Blood samples to obtain serum or EDTA-plasma for immediate processing, aliquoting and storage at − 80 °C, were collected on the day of admission to the ICU or study enrolment (between 0 and 7 days post-admission), and then daily up until day 7, day 10, day 14 and day 21. Samples were selected from day 1, 4, 7, 10, 14 and 21 where available. Healthy controls (n = 25) were drawn from a pre-existing biobank for baseline normative data with consideration, as best as possible, of age and sex. Demographic characteristics of each cohort are presented in Table S1. Timing of blood draws, clinical measurements (e.g., PaO2/FIO2 and serum creatinine [Cr]), comorbidities, and mortality of the patient cohort are presented in Table S2.
Lung function assessment
Determination of each patient's PaO2/FIO2 and static lung compliance were performed as previously reported [25].
Quantification of complement activation
Wieslab® Complement System Screen kits (Wieslab AB, Malmö, Sweden) were used to measure complement activation via the CP, the LP (MBL-dependent), and the AP in the serum of participants according to the manufacturer’s instructions. All determinations were performed in duplicate.
Serum and EDTA-plasma concentrations of complement activation products were measured using ELISA kits according to the manufacturers' instructions, with sample dilutions adjusted to fall within the linear range of standard curves. MicroVue™ EIA kits from Quidel Corporation (San Diego, USA) were used to measure C5a (cat #A025), sC5b-9 (cat #A029), Ba (cat #A033), Bb (cat #A027), and C4d (cat #A009). Hycult Biotech kits (Uden, NE) were used to measure FD (cat #HK343) and FH (cat #HK342). Serum levels of IL-6 were measured using the Simoa HD-1 platform (Quanterix Corporation, Billerica, USA), as previously reported [25]. Reported precision for each complement ELISA kit are listed as follows (analyte, coefficient of variation): C5a, ≤ 3.9%; sC5b-9, ≤ 6.8%; Ba, 2.3%; Bb, ≤ 4%; C4d, ≤ 9.7%. Precision characteristics were not available for FD and FH kits. All samples were tested in duplicate and only values with a CV of ≤ 15% were included in analyses.
Statistical analyses
Comparisons of two independent groups were performed using the Mann–Whitney U test. In each comparison, α < 0.05 was considered significant. Bars on scatterplots represent the median. Spearman’s rank correlation was used to evaluate associations between continuous variables. Correlative analyses were corrected for family-wise error using the Benjamini–Hochberg method with the false discovery rate set to 5%.
Simple logistic regression was used to investigate the effect of continuous and categorical variables on mortality within 30 days of ICU admission. Odds ratios and respective 95% confidence intervals (CI) were obtained to describe the odds of mortality based on a given predictor variable. Area under the receiver operator characteristic curve (ROC AUC) and classification rate were used to estimate model performance. McFadden’s pseudo-R squared was used to estimate goodness of fit of the maximum likelihood curve fitted to each model. p values were not considered, given the small sample size and exploratory nature of this analysis. To control for the effect of demographic variables on mortality, age and sex were tested alongside complement markers in multivariable logistic regressions, but given the low probability of an event (i.e., death within 30 days of ICU admission), the estimated minimum sample size required to reliably perform multivariable analyses was 118, using the formula \(\frac{10 \times k}{p}\), where k is the number of independent variables (2) and p is the probability of an event (0.17). The likelihood ratio test was performed to determine whether the inclusion of age or sex as predictor variables enhanced the fit of the data. In cases of complete separation between the categorical predictor variable and the outcome variable, the odds ratio and 95% CI were calculated using a 2 × 2 contingency table. All calculations were performed automatically by Prism 8 (GraphPad Software, San Diego, CA, USA).
Role of the funding source
The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Results
Increased activation of complement via the CP and AP in COVID-19 patients
The sera of 25 critically ill COVID-19 patients and a subset of 11 healthy controls were screened for ex vivo function of the 3 complement pathways by complement pathway-specific ELISA kits (Complement System Screen). Lower ex vivo activity reflects prior strong in vivo activation of the specific pathway with consumption of one or more key factors and consequently reduced residual capacity to activate complement via that pathway. In contrast to sera from healthy controls where expected complement levels were measured, we saw a marked reduction in residual capacity to activate complement via the CP in the sera of 72% of the COVID-19 patients and via the AP in 100% of the COVID-19 patients (Fig. 1). MBL-triggered complement activation in the sera of healthy controls was highly variable, consequently proportionate effects due to COVID-19 were insignificant.
In vitro complement pathway activity in serum from critically ill COVID-19 patients and healthy general population controls. Wieslab solid-phase screening assays were conducted to compare the residual complement pathway activity in healthy control (●) and COVID-19 patient (○) sera. All samples were tested in duplicate to obtain a mean
COVID-19 patients have elevated in vivo complement pathway activation biomarkers
To better delineate the pathways and factors involved, we measured serum concentrations of complement proteins and activation products in COVID-19 patients throughout their ICU stay. No significant sex-dependent differences in the concentrations of complement products were detected (Table S4). Compared to healthy controls, median levels of C5a and sC5b-9 in COVID-19 patients at the time of enrolment were 8.8-fold and 4.2-fold higher, respectively (p < 0.001 for both), indicating markedly increased activation of the terminal pathway (Fig. 2A, B) for all COVID-19 patients. We observed only a moderate association between serum sC5b-9 and C5a (rho = 0.48, p = 0.02), suggesting that circulating sC5b-9 and C5a levels vary somewhat independently, consistent with differences in their circulating half-lives and clearance. Given the feedback between coagulation and complement, which could potentially alter the between-group differences in complement activation products measured in serum, we assayed C5a and sC5b-9 in autologous EDTA-plasma from the 25 patients and a subset of controls. EDTA-plasma C5a and sC5b-9 were similarly significantly elevated in patients compared to controls, with similar effect sizes observed (Table S3). To further delineate the pathways involved, the C4b proteolytic fragment, C4d was measured. COVID-19 patient sera had significantly increased serum levels of C4d (3.1-fold higher, p < 0.0001), indicating elevated activation of the CP and/or the LP (Fig. 2C). Consistent with the functional Complement System Screen analyses, approximately 20% of COVID-19 patients had serum C4d levels that were within the normal range.
Markers of complement pathway activation or complement proteins in serum from healthy general population controls and critically ill COVID-19 patients admitted to the ICU. In all experiments, samples were tested in duplicate to obtain a mean. Prior to calculating protein concentrations, absorbance values were corrected for background absorbance of the detection system. Closed (●) and open (○) circles represent data for healthy controls and COVID-19 patients, respectively. Statistical significance was determined using the Mann–Whitney U test. p values were calculated automatically by Prism 8
The Complement System Screen data showed activation of the AP for all COVID-19 patients. This was further evidenced by significantly elevated serum antigenic levels of Ba and Bb as compared to healthy controls (Ba: 4.4-fold higher; Bb: 2.5-fold higher; p < 0.0001 for both; Fig. 2D, E). Since Ba and Bb may be increased via enhanced cleavage of FB by FD, we measured the latter by ELISA. A subset of COVID-19 patients had increased serum levels of FD, although the overall difference was not significant, further highlighting the phenotypic heterogeneity of COVID-19 patients (p = 0.23, Fig. 2F). Ba and Bb may also be elevated if there is increased formation or stability of the AP C3 convertase, caused in part, by reduced functional levels of the negative AP regulator, FH. Notably, serum levels of FH were significantly lower in the COVID-19 patients compared to healthy controls (p = 0.0062, Fig. 2G). Immunoblot analysis of patient sera did not reveal changes in the apparent molecular weight of FH under reducing conditions (Fig. S1), suggesting that major in vivo degradation of FH did not occur.
Renal dysfunction differentially affects levels of Ba and FD in patients with COVID-19
The major site of catabolism of both Ba and FD is the kidney [26, 27]. Thus, as might be predicted, circulating Ba and FD were elevated in patients with renal dysfunction [26, 28]. We tested whether renal dysfunction could explain the higher serum levels of Ba and FD in critically ill COVID-19 patients by comparing Ba and FD to serum creatinine (Cr), the latter being a measure of renal function. We observed strong positive correlations between Ba and FD (rho = 0.75, p < 0.0001), Ba and Cr (rho = 0.76, p < 0.0001), and FD and Cr (rho = 0.67, p < 0.0003). Patients were then stratified into two groups based on normal (Cr ≤ 100 mg/dL) or elevated serum Cr. Compared to healthy controls, COVID-19 patients with normal serum Cr still had significantly higher serum levels of Ba (Fig. 3A), but these Ba levels were significantly lower than those of the COVID-19 patients with elevated serum Cr (i.e. > 100 mg/dL). FD levels were similar between healthy controls and COVID-19 patients with normal serum Cr (p = 0.36, Fig. 3B), but significantly elevated in COVID-19 patients with renal dysfunction (serum Cr > 100 mg/dL). This suggested that increased FD in COVID-19 patients may reflect renal dysfunction that is linked to, but not solely responsible for increased Ba, and that a portion of the elevated Ba in critically ill COVID-19 patients arises from hyperactivation of the alternative pathway in vivo.
Serum Ba fragment and factor D in critically ill COVID-19 patients at the time of ICU admission separated by serum creatinine levels. Ba fragment (A) and factor D (B) concentrations in healthy general population controls compared to critically ill COVID-19 patients with clinically normal (≤ 100 mg/dL) or elevated (> 100 mg/dL) serum creatinine. Closed (●) circles, open (○) circles, and open diamonds (◊) represent data for healthy controls, COVID-19 patients, and patients with serum Cr > 100, respectively. Statistical significance was determined using the Mann–Whitney U test. P values were calculated automatically by Prism 8
Interestingly, in a subset of 11 COVID-19 patients with high serum levels of FD (> 1.5 µg/ml), there was a strong positive correlation between serum FD and FH (rho = 0.75, p = 0.009), which was not evident when all 25 patients were analyzed (rho = 0.24, p = 0.25) or when the remaining 14 patients with normal Cr were analyzed (rho = 0.25, p = 0.39) (Fig. S2). In spite of the higher FH levels in these patients, complement activation via the AP was heightened, as it was in the entire cohort of patients with COVID-19. This suggests that the expression/function of other negative regulators of complement may also be reduced in COVID-19 [13].
Elevated Ba and FD are predictive biomarkers for mortality of critically ill COVID-19 patients within 30 days post-ICU admission
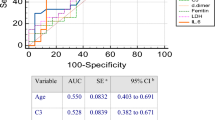
Elevated serum Cr has been shown to be a predictor of in-hospital mortality within 30 days [29, 30]. Given the strong positive correlations of serum Cr with Ba and FD, we similarly tested the ability of Cr, Ba, and FD to predict mortality in the 30 days following ICU admission in this cohort. Using a simple logistic regression model, we found serum Cr at the time of ICU admission was useful for discriminating between non-survivors and survivors, but elevated Cr at the time of ICU admission did not substantially affect the odds of dying (Table 1). By comparison, we found that both Ba and FD were useful for discriminating between non-survivors and survivors, they fit the dataset well, and a one unit increase of Ba or FD increased the odds of dying 4-5-fold (Table 1). Other complement analytes were also analyzed but none conveyed an increased odds of dying in this cohort. We also performed multivariable logistic regressions using Ba and age, Ba and sex, FD and age, or FD and sex as predictor variables (Table S5). These showed that neither age nor sex enhanced the power of Ba or FD in predicting mortality.
Similar to serum Cr, d-dimer has been proposed as a biomarker for the severity of COVID-19 and risk of mortality. Therefore, we compared the models for complement analytes to ICU admission levels of d-dimer. d-dimer was poor at discriminating between non-survivors and survivors, evidenced by the model performance, goodness of fit, and odds ratio (Table 1). This suggests that while d-dimer is useful for predicting death in cohorts comprising individuals with mild, moderate, and severe disease, it may not be useful for predicting death amongst critically ill patients, as shown here.
We also examined whether concentrations of inflammatory markers, respiratory status, and the presence of comorbidities at the time of ICU admission could be used to predict mortality. Consistent with other studies, only a previous diagnosis of hypertension, diabetes, or obesity appeared to increase the odds of dying in this cohort (Table 1).
Increased complement activation persists throughout hospitalization
In a prospective study of 39 COVID-19 patients, Holter et al. reported that complement activation persists for up to 10 days following hospitalization [3]. Similarly, we found that serum levels of C5a, sC5b-9, C4d, Ba, and Bb remained elevated over the 21-day study period. Time-course analyses of AP (Fig. 4) or terminal pathway (Fig. 5) components from individual patients showed that complement activation remained elevated but fluctuated in response to the administration of corticosteroids or the IL-6 receptor antagonist tocilizumab. Increased serum levels of FD and decreased levels of FH similarly persisted over the duration. The distributions of admission values showed considerable overlap with maximum and minimum recorded values (Fig. S3). Accordingly, the dynamic ranges of complement markers were substantially lower than those of acute-phase reactants IL-6, C-reactive protein (CRP), and ferritin (Fig. S4).
Serum concentrations of AP analytes FH, Ba, Bb, and FD throughout ICU stay for patients in which at least four time-points were available for testing. Patients who received the IL-6 receptor antagonist tocilizumab (B, C, E, F) were given a single dose of 400 mg on the day indicated. Shaded areas indicate the duration of daily corticosteroid administration for each patient where applicable. A Patient was treated with hydrocortisone on days 8–11, received 50 mg on day 13, and 25 mg on days 14–17. B Patient received 40 mg methylprednisolone daily from days 12–17 post-ICU admission. D patient received 100 mg hydrocortisone from days 12–16, 50 mg on day 17, and 100 mg from days 18–23. E Patient received hydrocortisone from days 2–6, day 8, and days 19–20. F Patient received hydrocortisone from days 7–16
Serum concentrations of terminal pathway analytes C5a and sC5b-9 throughout ICU stay for patients in which at least four time-points were available for testing. Patients who received the IL-6 receptor antagonist tocilizumab (B, C, E, F) were given a single dose of 400 mg on the day indicated. Shaded areas indicate the duration of daily corticosteroid administration for each patient where applicable. A Patient was treated with hydrocortisone on days 8–11, received 50 mg on day 13, and 25 mg on days 14–17. B patient received 40 mg methylprednisolone daily from days 12–17. D Patient received 100 mg hydrocortisone from days 12–16, 50 mg on day 17, and 100 mg from days 18–23. E Patient received hydrocortisone from days 2–6, day 8, and days 19–20. F Patient received hydrocortisone from days 7–16
Serum levels of C5a, Ba, and Bb are associated with poor respiratory function in critically ill COVID-19 patients
We previously reported a significant negative association between IL-6 levels and PaO2/FIO2 in this cohort of COVID-19 patients, with IL-6 being significantly elevated in those with ARDS [25]. Extending these studies to the complement system, correlation analyses revealed that significantly elevated serum concentrations of C5a, Ba, and Bb were negatively associated with PaO2/FIO2 in the COVID-19 patients on admission, with FD approaching significance (Table 2). The strength of each of these associations was similar to that of IL-6 [25]. Interestingly, there was a weak correlation between serum IL-6 with each of the serum levels of C5a, Ba, Bb, and FD (rho ≤ 0.25, p ≥ 0.24). There was also a negative correlation between Ba and Bb and static lung compliance on admission, measured in the 13 COVID-19 patients who were receiving invasive mechanical ventilation (n = 13, rho ≥ 0.6, p ≤ 0.042). sC5b-9 was the only complement analyte in serum that was significantly different between patients with and without ARDS, although the median difference between these groups was only ~ 20% (3 µg/ml; Table S6).
Discussion
In this report, we show that patients with severe COVID-19 all had increased activation of complement that persisted for at least 3 weeks from the time of ICU admission, and that ICU admission serum levels of Ba and FD were useful predictors of mortality in this cohort. Further, serum levels of AP components Ba and Bb and terminal pathway activation marker C5a inversely correlated with respiratory function, suggesting a relationship between the amount of AP activation and the severity of respiratory disease. These findings build on and are consistent with previous observations by other groups [3, 29,30,31,32,33,34], providing new insights into the important role that complement plays in COVID-19.
Although the mechanisms by which complement activation is triggered by SARS-CoV-2 remain poorly understood, recent studies are providing some interesting insights. Drawing from work with other highly pathogenic coronaviruses SARS-CoV-1 and MERS-CoV, the spike protein and nucleocapsid (N)-protein of SARS-CoV-2 can bind to MBL, ficolin-2 and collectin-11, thereby triggering activation of complement via the LP [32, 35, 36]. There is also a highly conserved motif within the N-protein of the highly pathogenic coronaviruses, including SARS-CoV-2, that binds to MASP-2 and triggers its autoactivation, resulting in enhanced generation of the LP C3 convertase in the presence of mannan and MBL [32]. Increased C4d levels in COVID-19 patients in our cohort and that of others, cannot distinguish the CP from the LP [3]. However, our Complement System Screen data implicate the CP in contributing to increased complement activation in most of our COVID-19 patients.
The mechanisms by which the CP participates in COVD-19 is less clear, but it is likely triggered by C1q recognition of anti-SARS-CoV-2 antibodies [3]. The CP may also be initiated in an antibody-independent manner via a direct interaction of the globular region of C1q with viral envelope glycoproteins shown for several viruses including, HIV-1, HTLV-1, EBV and CMV (reviewed in [37, 38]). To the best of our knowledge, these antibody-independent mechanisms of activation of the CP have not been definitively shown for SARS-CoV-2 or any of the other highly pathogenic coronaviruses. However, viral surfaces may be also recognized by serum amyloid peptide P, SIGN-R1 (a C-type lectin) and CRP, to which C1q can bind and trigger the CP [38]. In this regard elevated CRP levels, observed in our patient cohort, have been correlated with severity of COVID-19 [39] and may therefore contribute to CP activation.
Recent exciting advances reveal that complement activation is not restricted to the circulation, but may also occur within the cellular compartment, triggered by viruses and other pathogens (reviewed [40]). Thus, most complement components, including C3, C5 and FH, may be synthesized by immune and non-immune cells, including epithelial and endothelial cells. SARS-CoV-2 infection of respiratory epithelial cells has been shown to induce cleavage of intracellular C3, which may result in local release of C3a [41, 42]. Interestingly, generation of C3a by the virus-infected cells could not only be blocked by JAK 1/2 inhibitors, but also by a factor B inhibitor, the latter effect implicating an alternative-like pathway of activation [41]. The discovery of such intriguing cellular mechanisms of complement activation obviously have potential therapeutic implications and are worthy of further study.
Of the three complement pathways, it was only the AP, via which increased activation was observed in all of our COVID-19 patients. Recent studies have suggested that the SARS-CoV-2 spike protein may trigger activation of complement via the AP by competing with FH for cell-surface heparan sulfate, thus compromising its AP inhibitory role [4]. Changes in the function and/or quantity of other components of the AP might also contribute to heightened activation via the AP. In our COVID-19 cohort, elevated serum Ba and Bb levels were accompanied by a ~ 25% decrease in FH, a key negative regulator of the AP. The basis for the FH reduction is unknown, although it may be caused by increased clearance by the acute-phase reactant, CRP [43], the levels of which were elevated in our cohort of COVID-19 patients, as in others [30]. Nonetheless, this reduction in FH alone would not likely be sufficient to cause the thrombo-inflammatory response observed in COVID-19, but could contribute.
Our finding of sustained activation of complement in critically ill COVID-19 patients, has also been noted by others [3, 34]. These were extended by our studies by evaluating patients for up to 21 days. Even over that prolonged time period, complement activation persisted at a high steady level. This is notable, as the short circulating half-lives of the C3/C5 convertases and most of the complement activation products (e.g., Ba, Bb, and C5a) implies that, despite patients being discharged from the ICU to the ward and without obvious evidence of organ dysfunction, there is likely ongoing damage that is sustained or perpetuated by complement activation. Particularly in light of the relatively common occurrence of long-term and often serious sequelae post-SARS-CoV-2 infection, underlying complement activation may be clinically relevant. The fact that serum levels of other inflammatory markers, including IL-6, ferritin and CRP, varied widely in concentration and duration of elevation within and between patients, suggests that the complement pathway(s) may offer a more efficacious target for prevention/treatment of the "long-haul" post-acute syndrome, since biomarkers were persistently elevated in every patient.
We previously reported that IL-6 is significantly associated with severity of hypoxemia in our COVID-19 patient cohort [25], and some studies have shown efficacy in treating COVID-19 with the anti-IL-6 antibody tocilizumab [44,45,46]. In these COVID-19 patients, we show that Ba, Bb, and C5a were independently associated with lung function and that the associations with PaO2/FIO2 were as reliant as IL-6. Interestingly, although Ba, Bb, C5a, serum ferritin, CRP and IL-6 are each independently associated with PaO2/FIO2, there was no significant correlation between the markers of complement activation (Ba, Bb, C5a), and the acute-phase reactant markers of inflammation (IL-6, CRP, ferritin). This would imply that the influencing pathways are distinct. Such information may be of value in strategically designing combination therapies at different times in the course of the disease.
Other blood-borne biomarkers that are predictive of poor outcome have been identified by other groups. For example, multiple studies have concluded that elevated D-dimer is highly predictive of in-hospital mortality [29, 30, 47,48,49,50]. Our studies, however, did not reveal such an association, a discordance that is likely attributable to our small cohort size, and that our study was restricted to severely ill COVID-19 patients, but may indicate that complement biomarkers offer a more instructive correlation.
In spite of the small cohort, our between-group comparisons were effectively powered given the magnitude of differences of complement activation markers between patients and controls. However, logistic regression analyses were limited by sample size and may be underpowered. Larger cohort studies could further support the utility of Ba and FD as early predictors of mortality in COVID-19, and the current study serves as rationale. Small sample size also limited our ability to develop multivariable models of complement analytes to predict mortality as well as the performance of forward selection of additional variables to control for potential confounding effects of age, sex, and known comorbidities. Age-dependent changes in complement factors have been reported, but these observations only strengthen our findings, in that with age, circulating levels of FD decreased, while the complement inhibitor protein, C1-INH, increased [51]. The novelty of our findings also limited our ability to perform cross-validation of these models using other published datasets. Finally, although we attempted to consider age in the selection of our control cohort, strict age matching of patients to controls was not feasible, limiting our control of the effects of age-related changes in complement responses.
In summary, our data align very closely with others, in that complement activation in COVID-19 is markedly increased, that all pathways are likely involved, and that some activation markers have striking predictive value in terms of clinical outcomes [3]. Here we present the first study to report serum levels of Ba and FD in COVID-19, thereby providing additional insights into the pathways involved and the potential utility of these markers as predictors of disease and outcome. From a therapeutic perspective, there are numerous anti-complement therapies in preclinical and clinical development for COVID-19, none of which have yet yielded overwhelming benefit. This may be due to many factors, including for example, the dose and timing of administration, the complement activation site of intervention, the subset of patients being treated, and the use of concomitant therapies. Lack of major success by blocking the terminal pathway with a C5 inhibitor alone may be due to in part to the existence of C5 bypass pathways [52, 53]. Recent studies implicating the C5a–C5a receptor 1 (C5aR1) axis in the pathophysiology of COVID-19-associated acute lung injury and ARDS [54, 55], suggest that blockade of this pathway may be more beneficial [56,57,58]. Our data and that of others would support interfering with the AP by, for example, inhibiting FD. Most intriguing is the potential contribution of intracellular complement that may yield entirely new therapeutic strategies [41, 42]. Overall, our study highlights the involvement of complement in COVID-19, and provides strong rationale for continued efforts to identify predictive biomarkers of disease activity, and to delineate the underlying mechanisms toward the development of better treatments.
References
Lavine JS, Bjornstad ON, Antia R (2021) Immunological characteristics govern the transition of COVID-19 to endemicity. Science 371:741–745
Becker RC (2021) COVID-19 and its sequelae: a platform for optimal patient care, discovery and training. J Thromb Thrombolysis
Holter JC, Pischke SE, de Boer E, Lind A, Jenum S, Holten AR, Tonby K, Barratt-Due A, Sokolova M, Schjalm C, Chaban V, Kolderup A, Tran T, Gjølberg TT, Skeie LG, Hesstvedt L, Ormåsen V, Fevang B, Austad C, Müller KE, Fladeby C, Holberg-Petersen M, Halvorsen B, Müller F, Aukrust P, Dudman S, Ueland T, Andersen JT, Lund-Johansen F, Heggelund L, Dyrhol-Riise AM, Mollnes TE (2020) Systemic complement activation is associated with respiratory failure in COVID-19 hospitalized patients. Proc Natl Acad Sci USA 117:25018–25025
Yu J, Yuan X, Chen H, Chaturvedi S, Braunstein EM, Brodsky RA (2020) Direct activation of the alternative complement pathway by SARS-CoV-2 spike proteins is blocked by factor D inhibition. Blood 136:2080–2089
Noris M, Benigni A, Remuzzi G (2020) The case of complement activation in COVID-19 multiorgan impact. Kidney Int 98:314–322
Lo MW, Kemper C, Woodruff TM (2020) COVID-19: complement, coagulation, and collateral damage. J Immunol 205:1488–1495
Conway EM, Pryzdial EL (2020) Is the COVID-19 thrombotic catastrophe complement-connected? J Thromb Haemost 18:2812–2822
Danzi GB, Loffi M, Galeazzi G, Gherbesi E (2020) Acute pulmonary embolism and COVID-19 pneumonia: a random association? Eur Heart J 41:1858
Fox S, Akmatbekov A, Harbert J, Li G, Quincy Brown J, Vander Heide R (2020) Pulmonary and cardiac pathology in COVID-19: the first autopsy series from New Orleans. MedRxiv (preprint, not peer-reviewed)
Klok FA, Kruip M, van der Meer NJM, Arbous MS, Gommers D, Kant KM, Kaptein FHJ, van Paassen J, Stals MAM, Huisman MV, Endeman H (2020) Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res 191:145–147
Calabrese LH (2020) Cytokine storm and the prospects for immunotherapy with COVID-19. Cleve Clin J Med 87:389–393
Morgan BP (2000) The complement system: an overview. Methods Mol Biol (Clifton, NJ 150 (2000).
Ricklin D, Reis ES, Lambris JD (2016) Complement in disease: a defence system turning offensive. Nat Rev Nephrol 12:383–401
Barnum SR, Bubeck D, Schein TN (2020) Soluble membrane attack complex: biochemistry and immunobiology. Front Immunol 11:585108
Gasser C, Gautier E, Steck A, Siebenmann RE, Oechslin R (1955) Hemolytic-uremic syndrome: bilateral necrosis of the renal cortex in acute acquired hemolytic anemia. Schweiz Med Wochenschr 85:905–909
Hofer J, Rosales A, Fischer C, Giner T (2014) Extra-renal manifestations of complement-mediated thrombotic microangiopathies. Front Pediatr 2:97
Unsworth DJ (2008) Complement deficiency and disease. J Clin Pathol 61:1013–1017
Sim RB, Tsiftsoglou SA (2004) Proteases of the complement system. Biochem Soc Trans 32:21–27
Noris M, Remuzzi G (2005) Hemolytic uremic syndrome. J Am Soc Nephrol 16:1035–1050
Friese MA, Hellwage J, Jokiranta TS, Meri S, Peter HH, Eibel H, Zipfel PF (1999) FHL-1/reconectin and factor H: two human complement regulators which are encoded by the same gene are differently expressed and regulated. Mol Immunol 36:809–818
Alexander JJ, Quigg RJ (2007) The simple design of complement factor H: looks can be deceiving. Mol Immunol 44:123–132
Licht C, Pluthero FG, Li L, Christensen H, Habbig S, Hoppe B, Geary DF, Zipfel PF, Kahr WH (2009) Platelet-associated complement factor H in healthy persons and patients with atypical HUS. Blood 114:4538–4545
Delvaeye M, Noris M, De Vriese A, Esmon CT, Esmon NL, Ferrell G, Del-Favero J, Plaisance S, Claes B, Lambrechts D, Zoja C, Remuzzi G, Conway EM (2009) Thrombomodulin mutations in atypical hemolytic-uremic syndrome. N Engl J Med 361:345–357
Cofiell R, Kukreja A, Bedard K, Yan Y, Mickle AP, Ogawa M, Bedrosian CL, Faas SJ (2015) Eculizumab reduces complement activation, inflammation, endothelial damage, thrombosis, and renal injury markers in aHUS. Blood 125:3253–3262
Stukas S, Hoiland RL, Cooper J, Thiara S, Griesdale DE, Thomas AD, Orde MM, English JC, Chen LYC, Foster D, Mitra AR, Romano K, Sweet DD, Ronco JJ, Kanji HD, Chen Y-WR, Wong SL, Wellington CL, Sekhon MS (2020) The association of inflammatory cytokines in the pulmonary pathophysiology of respiratory failure in critically ill patients with coronavirus disease 2019. Crit Care Explor 2:e0203–e0203
Oppermann M, Kurts C, Zierz R, Quentin E, Weber MH, Gotze O (1991) Elevated plasma levels of the immunosuppressive complement fragment Ba in renal failure. Kidney Int 40:939–947
Jalal D, Renner B, Laskowski J, Stites E, Cooper J, Valente K, You Z, Perrenoud L, Le Quintrec M, Muhamed I, Christians U, Klawitter J, Lindorfer MA, Taylor RP, Holers VM, Thurman JM (2018) Endothelial microparticles and systemic complement activation in patients with chronic kidney disease. J Am Heart Assoc 7:e007818
Volanakis JE, Barnum SR, Giddens M, Galla JH (1985) Renal filtration and catabolism of complement protein D. N Engl J Med 312:395–399
Cheng Y, Luo R, Wang K, Zhang M, Wang Z, Dong L, Li J, Yao Y, Ge S, Xu G (2020) Kidney disease is associated with in-hospital death of patients with COVID-19. Kidney Int 97:829–838
Tanboğa IH, Canpolat U, Çetin EHÖ, Kundi H, Celik O, Cağlayan M, Ata N, Özeke Ö, Çay S, Kaymaz C, Topaloğlu S (2021) Development and validation of clinical prediction models to estimate the probability of death in hospitalized patients with COVID-19: insights from a Nationwide Database. J Med Virol 93:3015–3022
Cugno M, Meroni PL, Gualtierotti R, Griffini S, Grovetti E, Torri A, Panigada M, Aliberti S, Blasi F, Tedesco F, Peyvandi F (2020) Complement activation in patients with COVID-19: a novel therapeutic target. J Allergy Clin Immunol 146:215–217
Gao T, Hu M, Zhang X, Li H, Zhu L, Kuiu H, D. Q, Zhang Z, Wang Z, Hu Y, Fu Y, Jin Y, Li K, Zhao S, Xiao Y, Luo S, Li L, Zhao L, Liu J, Zhao H, Liu Y, Yang W, Peng J, Chen X, Li P, Liu Y, Xie Y, Song JJ, Zhang L, Ma Q, Bian X, Chen W, Liu X, Mao Q, Cao C (2020) Highly pathogenic coronavirus N protein aggravates lung injury by MASP-2-mediated complement over-activation. MedRxiv (preprint, not peer-reviewed)
Sinkovits G, Mezo B, Reti M, Muller V, Ivanyi Z, Gal J, Gopcsa L, Remenyi P, Szathmary B, Lakatos B, Szlavik J, Bobek I, Prohaszka ZZ, Forhecz Z, Csuka D, Hurler L, Kajdacsi E, Cervenak L, Kiszel P, Masszi T, Valyi-Nagy I, Prohaszka Z (2021) Complement overactivation and consumption predicts in-hospital mortality in SARS-CoV-2 infection. Front Immunol 12:663187
de Nooijer AH, Grondman I, Janssen NAF, Netea MG, Willems L, van de Veerdonk FL, Giamarellos-Bourboulis EJ, Toonen EJM, Joosten LAB, R.-C.-s. group (2021) Complement activation in the disease course of coronavirus disease 2019 and its effects on clinical outcomes. J Infect Dis 223:214–224
Ip WK, Chan KH, Law HK, Tso GH, Kong EK, Wong WH, To YF, Yung RW, Chow EY, Au KL, Chan EY, Lim W, Jensenius JC, Turner MW, Peiris JS, Lau YL (2005) Mannose-binding lectin in severe acute respiratory syndrome coronavirus infection. J Infect Dis 191:1697–1704
Ali YM, Ferrari M, Lynch NJ, Yaseen S, Dudler T, Gragerov S, Demopulos G, Heeney JL, Schwaeble WJ (2021) Lectin pathway mediates complement activation by SARS-CoV-2 proteins. Front Immunol 12:714511
Thielens NM, Tacnet-Delorme P, Arlaud GJ (2002) Interaction of C1q and mannan-binding lectin with viruses. Immunobiology 205:563–574
Agrawal P, Nawadkar R, Ojha H, Kumar J, Sahu A (2017) Complement evasion strategies of viruses: an overview. Front Microbiol 8:1117
Ji SR, Wu Y, Potempa LA, Liang YH, Zhao J (2006) Effect of modified C-reactive protein on complement activation: a possible complement regulatory role of modified or monomeric C-reactive protein in atherosclerotic lesions. Arterioscler Thromb Vasc Biol 26:935–941
Arbore G, Kemper C, Kolev M (2017) Intracellular complement—the complosome—in immune cell regulation. Mol Immunol 89:2–9
Yan B, Freiwald T, Chauss D, Wang L, West E, Mirabelli C, Zhang CJ, Nichols EM, Malik N, Gregory R, Bantscheff M, Ghidelli-Disse S, Kolev M, Frum T, Spence JR, Sexton JZ, Alysandratos KD, Kotton DN, Pittaluga S, Bibby J, Niyonzima N, Olson MR, Kordasti S, Portilla D, Wobus CE, Laurence A, Lionakis MS, Kemper C, Afzali B, Kazemian M (2021) SARS-CoV-2 drives JAK1/2-dependent local complement hyperactivation. Sci Immunol 6:abg0833
Yan B, Freiwald T, Chauss D, Wang L, West E, Bibby J, Olson M, Kordasti S, Portilla D, Laurence A, Lionakis MS, Kemper C, Afzali B, Kazemian M (2020) SARS-CoV2 drives JAK1/2-dependent local and systemic complement hyper-activation. Res Sq. https://doi.org/10.21203/rs.3.rs-33390/v1
Okemefuna AI, Nan R, Miller A, Gor J, Perkins SJ (2010) Complement factor H binds at two independent sites to C-reactive protein in acute phase concentrations. J Biol Chem 285:1053–1065
Yuan J, Zou R, Zeng L, Kou S, Lan J, Li X, Liang Y, Ding X, Tan G, Tang S, Liu L, Liu Y, Pan Y, Wang Z (2020) The correlation between viral clearance and biochemical outcomes of 94 COVID-19 infected discharged patients. Inflamm Res 69:599–606
Zhang C, Wu Z, Li JW, Zhao H, Wang GQ (2020) Cytokine release syndrome in severe COVID-19: interleukin-6 receptor antagonist tocilizumab may be the key to reduce mortality. Int J Antimicrob Agents 55:105954
Xu X, Han M, Li T, Sun W, Wang D, Fu B, Zhou Y, Zheng X, Yang Y, Li X, Zhang X, Pan A, Wei H (2020) Effective treatment of severe COVID-19 patients with tocilizumab. Proc Natl Acad Sci USA 117:10970–10975
Muhammad R, Ogunti R, Ahmed B, Munawar M, Donaldson S, Sumon M, Kibreab A, Thomas AN, Mehari A (2021) Clinical characteristics and predictors of mortality in minority patients hospitalized with COVID-19 infection. J Racial Ethnic Health Disparities. https://doi.org/10.1007/s40615-020-00961-x
Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, Xiang J, Wang Y, Song B, Gu X, Guan L, Wei Y, Li H, Wu X, Xu J, Tu S, Zhang Y, Chen H, Cao B (2020) Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 395:1054–1062
Zhang L, Yan X, Fan Q, Liu H, Liu X, Liu Z, Zhang Z (2020) D-dimer levels on admission to predict in-hospital mortality in patients with COVID-19. J Thromb Haemost 18:1324–1329
Li Y, Zhao K, Wei H, Chen W, Wang W, Jia L, Liu Q, Zhang J, Shan T, Peng Z, Liu Y, Yan X (2020) Dynamic relationship between d-dimer and COVID-19 severity. Br J Haematol 190:e24–e27
Da Costa MG, Poppelaars F, Van Kooten C, Mollnes TE, Tedesco F, Würzner R, Trouw LA, Truedsson L, Daha MR, Roos A, Seelen MA (2018) Age and sex-associated changes of complement activity and complement levels in a healthy caucasian population. Front Immunol 9:1–14
Masaki T, Matsumoto M, Yasuda R, Levine RP, Kitamura H, Seya T (1991) A covalent dimer of complement C4b serves as a subunit of a novel C5 convertase that involves no C3 derivatives. J Immunol 147:927–932
Mannes M, Dopler A, Zolk O, Lang SJ, Halbgebauer R, Hochsmann B, Skerra A, Braun CK, Huber-Lang M, Schrezenmeier H, Schmidt CQ (2021) Complement inhibition at the level of C3 or C5: mechanistic reasons for ongoing terminal pathway activity. Blood 137:443–455
Chouaki Benmansour N, Carvelli J, Vivier E (2021) Complement cascade in severe forms of COVID-19: recent advances in therapy. Eur J Immunol
Carvelli J, Demaria O, Vely F, Batista L, Chouaki-Benmansour N, Fares J, Carpentier S, Thibult ML, Morel A, Remark R, Andre P, Represa A, Piperoglou C, C.-I.P.H.g. Explore, C.-M.I.g. Explore, Cordier PY, Le-Dault E, Guervilly C, Simeone P, Gainnier M, Morel Y, Ebbo M, Schleinitz N, Vivier E (2020) Association of COVID-19 inflammation with activation of the C5a–C5aR1 axis. Nature 588:146–150
Woodruff TM, Shukla AK (2020) The complement C5a–C5aR1 GPCR axis in COVID-19 therapeutics. Trends Immunol 41:965–967
Posch W, Vosper J, Noureen A, Zaderer V, Witting C, Bertacchi G, Gstir R, Filipek PA, Bonn GK, Huber LA, Bellmann-Weiler R, Lass-Florl C, Wilflingseder D (2021) C5aR inhibition of nonimmune cells suppresses inflammation and maintains epithelial integrity in SARS-CoV-2-infected primary human airway epithelia. J Allergy Clin Immunol 147:2083-2097.e6
Jiang Y, Zhao G, Song N, Li P, Chen Y, Guo Y, Li J, Du L, Jiang S, Guo R, Sun S, Zhou Y (2018) Blockade of the C5a–C5aR axis alleviates lung damage in hDPP4-transgenic mice infected with MERS-CoV. Emerg Microbes Infect 7:77
Funding
EMC was supported by grants from CanVECTOR (COVID-19 Rapid Response Funding Competition), the Canada Research Chairs program, the Canadian Institutes of Health Research (#407306), and Fastgrants.org. EMC and ELGP also received support from the Heart and Stroke Foundation of Canada (EMC—G-18-0022122; ELGP—G-19-0026524). AL and HW were supported by Mitacs Accelerate Fellowships (IT19262, awarded to EMC and ELGP).
Author information
Authors and Affiliations
Contributions
EMC, ELGP, CLW, and MSS were responsible for the conception and design of the study. AL, SS, VL, and HW designed and performed experiments and analyzed data. RLH and JC collected and analyzed data. AL, EMC, and ELGP led the manuscript writing, which was critically reviewed by CJC, CLW, SS, and MSS. MSS, CLW, and SS provided access to essential materials and information. All authors approved the final version of the manuscript. EMC and ELGP supervised the study.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to disclose.
Additional information
Edited by: Jörg Köhl.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Leatherdale, A., Stukas, S., Lei, V. et al. Persistently elevated complement alternative pathway biomarkers in COVID-19 correlate with hypoxemia and predict in-hospital mortality. Med Microbiol Immunol 211, 37–48 (2022). https://doi.org/10.1007/s00430-021-00725-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00430-021-00725-2