Abstract
Purpose of Review
Human-caused global change is fundamentally altering natural forest ecosystems. More trees are exhibiting a wide range of symptoms indicative of poor vigour, particularly stressed species at the edge of their native ranges and stands growing on marginal sites. This review will focus on complex tree diseases (declines) caused by native pathogens and the key environmental drivers that contribute to this phenomenon. These systems are frequently complex, with multiple drivers at work.
Recent Findings
Using four cases studies on different continents, we explored the direct and indirect environmental drivers underlying these decline syndromes. Although climate and weather events seem to be usually associated with forest decline, we found that environmental disturbance by either forest management or land-use changes is also a global predisposing factor of decline which deserves more attention. Changes in land use have directly benefited pathogens such as root rots in the Pyrenees (Spain) or indirectly by making the environment more conducive for canker and foliar diseases in Australia and the USA. Focus on land-use changes could improve understanding of current decline problems such as those affecting Araucaria in Chile.
Summary
The next century will almost certainly see an unprecedented rise in forest pathogen epidemics, requiring a proactive rather than reactive response. Diseases caused by native pathogens with complex aetiologies will become more common, and recognising, characterising and managing these epidemics are difficult because native pathogens are frequently already widespread, and eradication is not feasible. We need to start approaching these issues from a ‘whole ecosystem’ perspective, highlighting the many aspects and entanglements of forest declines and allowing us to respond with management options tailored to each scenario. The approach proposed here provides logical steps based on six questions to untangle the direct and indirect environmental drivers of tree declines.
Similar content being viewed by others
Introduction
Forest systems are experiencing unprecedented transformation rates due to many disruptions related to global change, including land clearing and fragmentation, severe droughts and wildfires and outbreaks of invasive alien pests and pathogens [1]. Several reviews have explored these topics. However, one gap in the current literature is exploring how anthropogenic disturbances lead to the emergence of native pathogens resulting in disease epidemics more typical of invasive alien pathogens. Understanding and teasing out the drivers of native epidemics in forest ecosystems will aid in mitigating this problem. As eradicating ubiquitous native pathogens is not an option, management strategies must focus on ameliorating ecosystem stress, a complex undertaking.
Disease is a consequence of a virulent pathogen, a susceptible host and a conducive environment (i.e. the disease triangle [2]). Trees in natural ecosystems are mixed in age and species composition, and the impact of pathogens adapted to a particular host or life stage is limited by the availability of resources [3]. Natural ecosystems are, typically, in balance with their native pathogens, and severe disease outbreaks are rare [4]. However, this co-evolution occurred over time and within an environmental envelope [5]. Human activities have disturbed this natural balance between trees and their co-evolved pathogens, reducing ecosystem resilience. These activities include land clearing and fragmentation, leading to more edges exposed to climatic fluctuations and other stressors such as increased run-off or pesticides from agriculture. Harvesting of trees, selective or clear-felling, alters age class and species composition, and hence the availability of susceptible hosts. This complex interplay between natural resilience and increased stressors can lead to native pathogens causing disease epidemics as impactful as those typically seen for invasive pathogens. In addition to these direct impacts by human activities, the impacts of anthropogenic climate change are increasingly experienced in forest systems, with this further contributing to disease development by native pathogens.
The study of emerging infectious diseases has its history in medical and veterinary fields but has also applied to plant pathogens [6]. Three barriers limit the development of emerging infectious diseases: geographical, environmental and evolutionary (compatibility) (for detail, see Paap et al. [7]). Release from any of these barriers can result in disease development. In the context of invasive alien pathogens, globalisation (trade leading to accidental movement of forest pathogens to naïve suitable hosts) allows pathogens to overcome geographical barriers [8]. If this leads to opportunities for microorganisms to encounter naïve hosts lacking co-evolved resistance, then the evolutionary/compatibility barriers are crossed. There are numerous examples of this: sudden oak death in the western USA [9], chestnut blight [10], Phytophthora dieback in the southwest of Australia [11], Dutch elm disease [12] and Ash dieback [13].
Pertinent to this review, native pathogens do not need to overcome geographical or evolutionary boundaries. However, the environmental barrier may be overcome due to land-use change and/or overarching climate change, leading to situations where native pathogens exhibit increased pathogenicity to co-evolved hosts. For example, selective logging or clear-felling coups can result in an even-aged monoculture of a native tree species that is now equally susceptible to specific pathogens [14••, 15]. The environment can alter host–pathogen relationships, and depending on the region, climatic conditions may become more or less favourable for pathogens. Weather (short-term atmospheric conditions) and climate (weather of a specific region averaged over a long time period) both impact pathogens, their hosts and their interactions. Climate change refers to long-term changes in climate. For example, climate change leading to long-term drying is a chronic change while drought is an acute change; both may favour pathogens by enhancing their fitness or driving expansion in distribution ranges and/or exerting stress on tree hosts, predisposing them to disease development [16••].
Perhaps, the first to highlight the role of climate in forest disease was Hepting [17], who concluded that a severe outbreak of a native pathogen ‘is a rare removal by the weather of obstacles that ordinarily constrain the pathogen’. In an era of global change, the disease triangle must be updated to consider the environmental component in two timeframes: the weather, which must be conducive to disease development, and overall climate change and the underlying stress it causes [18]. The disease triangle only explains the point at which disease is initiated and the subsequent symptoms that develop (acute); other models (such as Manion’s decline spiral) treat tree disease in a more holistic space taking into account other interactions of the host and pathogens. The concept of chronic tree disease or tree decline caused by interchangeable predisposing, inciting and contributing factors was first proposed by Sinclair [19]. Manion [20] developed this concept further and proposed the tree disease spiral and later the decline disease spiral [21]. The early 2000s saw a rise in studies and reviews of challenges to forest health in the face of globalisation and climate change [6, 22, 23]. Drought, in particular, was seen as a significant driver of disease emergence.
The impact of invasive alien forest pathogens is well documented; this review will concentrate on complex tree diseases (declines) caused by native pathogens in the era of anthropogenic global change. As elucidated by three case studies, we explore the key environmental drivers behind this phenomenon, showing that these systems are often complex, with multiple drivers involved. Finally, using the decline of Araucaria araucana in South America as an example, we recommend an experimental approach that could be applied to understand the dynamics of emerging forest pathogen outbreaks. We highlight the need for this approach to consider both temporal and multi-spatial scales.
Marri Canker; Pathogen: Quambalaria coyrecup, Host: Corymbia calophylla
Aetiology and Extent
Quambalaria coyrecup is a basidiomycete smut-like fungus thought to be endemic to the southwest of Western Australia (SWWA), and it is the causative agent of stem cankers in Corymbia calophylla, a keystone Myrtaceae tree species endemic to SWWA [24]. Excessive bleeding (gummosis), staining limbs or trunks dark red, splitting and eventual shedding of bark revealing perennial cankers and Q. coyrecup sporulation as powdery white masses within the cankered area are all symptoms of this disease [25]. As cankers progress, they eventually ‘girdle’ the affected limb or trunk, resulting in the death of the limb or death of the entire tree (Fig. 1). These symptoms were first observed in C. calophylla in 1939–1940, but at the time were of no serious concern. In subsequent decades, additional biotic and abiotic factors have caused changes in this native pathogen-host relationship. Such factors include climate change, increases in habitat fragmentation and interactions with invasive plant pathogens. By the 1990s, these stem cankers were causing a noticeable decline of C. calophylla.
Environmental Drivers
The SWWA is a highly fragmented landscape with increasing pressure from expansion in both agriculture and urbanisation [26]. This habitat loss has resulted in forest fragments interspersed amongst various land-use types [27]. Forest fragments have distinct edge habitats that experience different environmental conditions compared to the interior of fragments [28,29,30]. Specifically, highly fragmented areas in SWWA have resulted in remnant tree stands dominated by C. calophylla, and these fragmented areas are surrounded by different types of land use (e.g. road and land used for horticulture, agriculture, viticulture or grazing). It is in these highly fragmented areas that canker incidence is highest. Forest edges only exposed to one type of land use, and interiors of forest fragments have lower canker incidence than highly fragmented areas [31••].
The SWWA has experienced dramatic climate changes. Rainfall has declined over the past 30 years, and in combination with a growing population requiring increases in water demand, groundwater levels have declined [32]. The region has also experienced heatwave-compounded drought events [33]. With both long-term precipitation reduction and drought, SWWA has experienced long-term drying, the impacts of which have already been observed in crown dieback in dominant forest species, Eucalyptus marginata and C. calophylla [34]. This overall climate change is one of the abiotic factors driving canker incidence in C. calophylla.
Indirect Environmental Drivers
Anthropogenic disturbance also changes soil microclimate, which results in alterations in soil chemistry and composition belowground [35•] and plant diversity aboveground [31••]. Belowground processes and communities are highly sensitive to these changes, further predisposing C. calophylla to canker disease. For example, concentrations of key soil nutrients (i.e. nitrogen, phosphorus and potassium) are much higher along edge habitats where C. calophylla has a higher canker incidence [35•]. In addition, communities of beneficial mycorrhizal fungi have lower richness and diversity in edge habitats in comparison to forest habitats [35•].
Fragmented forests can experience greater pressure from invasive plant pathogens [36]. Phytophthora spp. have caused devastation to forests in SWWA, especially the invasive Phytophthora cinnamomi [37]. Landscape-scale surveys identified that C. calophylla present on sites infested with pathogenic Phytophthora species had higher levels of canker incidence [38]. Glasshouse studies also showed C. calophylla co-infected with Phytophthora spp. and Q. coyrecup were more stressed than C. calophylla infected with Q. coyrecup only [39]. To add to the complexity, the loss of ectomycorrhizal fungal diversity could play a role in Phytophthora spp. infection [40]. Together, these changes in abiotic and biotic characteristics of the soil are highly correlated with increased disease incidence in C. calophylla [35•].
Aboveground, anthropogenic disturbance has altered forest stand structure where highly fragmented areas have different overstory composition than the interior of forest fragments [31••]. Interior forest fragments consist of mixed E. marginata and C. calophylla overstory; E. marginata may be less tolerant to fragmentation as this species is not present in highly fragmented areas (however, E. marginata is removed by selective logging practices). In either case, the loss of diversity in highly fragmented areas may have led to levels of pathogen pressure of Q. coyrecup above those which C. calophylla is adapted to in their natural forest setting [41].
The impacts of anthropogenic disturbance on C. calophylla health can also interact with climate and drought events. Climate plays a major role in the development of cankers in C. calophylla. For example, cankers have been observed across most of the range of C. calophylla, but areas with wetter and cooler climates have high incidences of canker [38]. The timing and length of droughts within these environments are also important in disease development [42].
Summary
Overall, the decline of C. calophylla seems to have been instigated by anthropogenic disturbance but is a complicated decline involving changes both above- and belowground. Specifically, a drying climate combined with habitat fragmentation and the indirect effects of habitat fragmentation (i.e. changes in soil nutrients and biotic communities) predisposes C. calophylla to canker disease caused by Q. coyrecup.
Silver Fir Decline; Pathogens: Various, Host: Abies alba
Aetiology and Extent
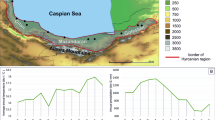
The health of silver fir (Abies alba) in the Pyrenees (mountain range straddling the border of France and Spain) has been of concern for the last three decades (Fig. 2). Several stands in the southern side of the Pyrenees started showing signs of decline associated with the severe droughts occurring in the 1980s [43]. A large-scale pathological systematic sampling revealed a high incidence of root rots such as Heterobasidion annosum (42% of the stands) and Armillaria spp. (93%), canker pathogens such as Melampsorella caryophyllacearum (55% of the stands) or parasitic plants such as Viscum album [44, 45••]. At a Pyrenean scale, the parasitic plant Viscum album was the biotic agent most clearly associated with symptoms of decline [45••]. Concerning the root rots, there is no information on the severity of those pathogens and how much they increased in association with the decline process; however, the observed incidences (> 40%) are far greater than those observed in natural forests [46]. Later work on specific pathogens showed that both H. annosum s.s. and H. abietinum affected fir stands, although the latter was far more abundant [47]. Several species of Armillaria were also found affecting fir forests, with A. ceptistipes being the most abundant and the one associated with higher defoliation. Aggressive Armillaria species such as A. ostoyae or A. gallica were more associated with fir-accompanying species such as Pinus uncinata or Fagus sylvatica than with fir decline [48].
Later studies in more localised declining stands revealed high local severities of pathogens such as Amylostereum chailletii and Trichaptum abietinum, together with the above-mentioned root rots [49••]. Some interactions between the identified pathogens were found. For instance, V. album was more prevalent in forests attacked by H. abietinum [47]. Other secondary pests such as bark beetles from the genus Pityokteines have been associated with mortality in declining forests [45••].
Environmental Drivers
Fir forests were subjected to major logging during the second half of the twentieth century. During the selective logging process, some trees were left, and this caused massive regeneration underneath. Thereafter, most of the fir stands remained untouched, and encroachment and competition were paramount. Thus, the most common forest structure comprises two cohorts [45••], with trees belonging to the older cohort showing the most symptoms of decline. In most cases, the current land use involves a lower anthropogenic pressure than in the past, allowing the forest to expand to areas previously used as grassland or crop areas [50]. Across the Pyrenees, the abundance of Armillaria and Heterobasidion in dead trees and stumps and the abundance of Armillaria in soil were associated with higher intensity logging [47, 48]. The current prevalence of Heterobasidion is possibly inherited from the previous land use as no stump protection methods such as urea, Phlebiopsis gigantea or borate were applied at that time. Viscum album showed a similar association with former management practices, more prevalent on those isolated trees with large diameter and opened crowns [47]. There is no information on the association of Amylostereum with forest fragmentation in the context of fir decline. However, it is known that wasps carrying the pathogen affect stressed trees [51]; therefore, the presence of the pathogen is probably associated with trees left after the logging, the ones suffering the most considerable growth losses over the last century [52].
The Pyrenean population of silver fir is the southernmost distribution of the species in Eurasia. The Pyrenees have been subjected to major shifts in temperature and precipitation, leading to increased drought and the decline of different species. Silver fir in the Pyrenees has experienced a series of droughts [53], much more severe than in other populations in the Alps or Romania [54].
Indirect Environmental Drivers
Drought alone could not explain the phenomenon of decline and it was clear that it involved the effect of previous management. Declining stands showed many more growth releases than non-declining stands [43]. Management did not affect all species similarly, and some species growing near silver fir stands, such as Pinus sylvestris, benefited from it [55].
Another critical aspect is the selection of trees to remain after logging. Trees showing symptoms of decline in the 1980s were those growing slower at the beginning of the century and before harvesting in the second half of the century [49••]. Harvesting extracted the best and most vigorous trees and left those suppressed or co-dominant, probably with less capacity to adapt to the increased density and competition with the new cohort for the scarce water supplies [45••]. Management triggered structural changes that contributed to forest decline appearing decades after.
Summary
Evidence shows that logging was the predisposing factor for the decline of silver fir in the Pyrenees. Logging during the second half of the twentieth century favoured root rot pathogens and dramatically changed the structure of the forest, leaving the weaker trees remaining in the stands. After harvesting, forest management mainly was stopped, and encroachment and high tree-to-tree competition caused stress to the remaining trees. Amongst those, decline affected co-dominant and suppressed trees left from the previous rotation. The droughts in the late 1980s were an inciting factor for the decline, and it was then when the crown condition of fir started deteriorating. Since then, mortality has affected the largest trees, where secondary pests and pathogens contribute to tree death.
Caliciopsis Canker; Pathogen: Caliciopsis pinea, Host: Pinus strobus
Aetiology and Extent
Pinus strobus (eastern white pine) will naturally regenerate and tolerate a range of soil types and is utilised commercially for lumber. Caliciopsis pinea is a canker pathogen of P. strobus native to North America, with fruiting bodies of C. pinea first reported in New York in 1880 [56]. Subsequently, Caliciopsis canker disease was reported as a ‘minor’ canker disease with a ubiquitous distribution throughout the range of P. strobus [57]. Reports of Caliciopsis canker on P. strobus have increased in severity and distribution in the last 20 years, particularly in the northeastern USA (New England) and southeastern USA (the Southern Appalachian Mountains) [58••, 59••], where regeneration and planting strategies have led to dense stands of P. strobus. The disease is characterised by excessive resin production and reddish sunken cankers on stems and branches as well as bark fissures, crown thinning and dieback (Fig. 3) [58••]. Serious canker disease and declining P. strobus have now been observed in the Great Lakes region of Michigan and Wisconsin, and recent surveys (2018–2019) have highlighted the extensive distribution of the disease in these states. Caliciopsis pinea is a threat to P. strobus throughout its native range, and the risk it poses to other hosts remains unquantified [58••].
Environmental Drivers
Management decisions facilitating the growth of P. strobus on poor quality sites combined with a subsequent lack of management resulting in dense even-aged stands have led to favourable conditions for the emergence of the native C. pinea. More specifically, replanting and regenerating P. strobus on logged and abandoned agricultural and pastoral land, coupled with a lack of management and fire suppression, has led to overstocked, even-aged stands on compacted and plough pan soils that restrict root penetration [60, 61]. Together, these factors are driving the emergence of C. pinea. All age classes of P. strobus can be impacted by Caliciopsis canker, and the disease has been associated with regeneration (seedlings and saplings), pole timber mortality and sawtimber degradation [58••, 62].
Seasonal projections forecast warmer, wetter springs and winters coupled with dryer, hotter summers [63, 64]. Collectively, these climate changes lead to conditions favourable to pathogen reproduction and dissemination in the spring, improved overwintering survival of pathogens and increased water stress in the host during the summer [65••]. Increased temperature and precipitation has been linked to the emergence of needle cast pathogens [66], and increased temperatures have been linked to increased severity of Caliciopsis canker disease [59••].
Indirect Environmental Drivers
Pinus strobus is widely distributed across eastern America on many soil types. Disease severity and incidence have not been associated with ecoregions, plant hardiness zones, slope or aspect [58••, 59••]. Compared to well-drained, more fertile soils, disease incidence increased on sites with excessively or poorly drained soils, and disease severity increased on sites with nutrient-poor, excessively drained soils [58••]. Disease incidence and severity were also higher in drier and shallow soils when compared to loamy soils [67].
Costanza et al. [68•] uncovered regional synchrony in the timing of canker incidence and severity across different sites; tree declines triggered by climate stress events (i.e. drought and flooding) occurred 1–3 years before canker development. Disease severity is also positively associated with mean annual temperature [59••]. Climate change likely has wide-ranging impacts across broad geographical regions, and local differences in incidence and severity may be exacerbated by poor site and stand conditions.
Other biological pests and pathogens such as a native scale insect and a complex of native foliar fungi are thought to compound climate, stand and site stresses. The herbivorous insect Matsucoccus macrocicatrices pierces P. strobus bark and feeds on the sap, creating numerous wounds that are thought to add stress to the tree and facilitate the entry of C. pinea [69]. However, M. macrocicatrices has only recently been associated with C. pinea cankers [69, 70] and was previously considered benign. Therefore, the association between Caliciopsis canker disease and M. macrocicatrices is unclear. White pine needle pathogens (Lophophacidium dooksii, Lecanosticta acicola and Bifusella linearis) are native foliar fungi that cause premature needle loss [71], with disease severity linked to warmer and wetter seasons [61, 66], with associated host mortality exacerbated by site and stand characteristics and the presence of Caliciopsis canker disease [68•].
Summary
Overstocking, deficient soil conditions (poor drainage, nutrient-poor, shallow), pests and pathogens are linked with increased Caliciopsis canker disease incidence and severity. Younger, smaller trees are more impacted than larger trees, and inadequate soil water and excessively shallow-rooted trees are more likely to be impacted during drought events. In general, selecting sites with deep, sandy, well-drained soils and stand thinning at prescribed size classes is recommended to increase tree vigour, resilience and resource availability [72]. Caliciopsis canker has now been observed in the Great Lakes region in Michigan and Wisconsin. Disease severity is currently lower than observed in the eastern states, and current research focuses on documenting the distribution, impact and epidemiology in the Great Lakes region.
Araucaria Decline: an Emerging Disease of Natural Forests in South America
A newly documented decline of Araucaria araucana (Araucaria, Pewén or the Monkey Puzzle tree) emerged between 2015 and 2016 (Fig. 4). This tree species is endemic to the south-central Andes Mountain range slopes in Chile and Argentina and the coastal range of Chile. Field surveys undertaken inside its natural range at different locations identified symptomatic trees in all visited areas. A broad initial description of symptoms consistently identified dieback of portions of the crown of trees with the death of a small proportion of young trees (between 1.5 and 3 m), and, in rare cases, death of full adult trees [73, 74]. Knowledge of this new disease is still scarce, and the factors driving its emergence are not understood. Surveys at different sites (latitudes) across the Andes distribution have shown the most prevalent symptoms to be cankers on branches and stems. These cankers slowly girdle healthy-looking branches and stems, causing the death of the affected organs. A fungus residing in a novel genus and species, Pewenomyces kutranfy, was found to be the causal agent of these cankers [74]. This pathogen is believed to be native as it has not been found elsewhere and is clearly adapted to the cold temperature characteristic of high-altitude environments. While evidence suggests this fungal canker disease is the prevalent disease associated with the dieback observed in A. araucana in the Andes [74], sites exist where cankers are only scarcely observed and where other abiotic factors and biotic agents seem predominant [75]. The observation of both cankers and other symptoms suggests that different disease syndromes may be present in different sites (populations).
This decline may be driven by changes in weather, including several years of intense drought [76], a rise in maximum and minimum temperatures and a reduction in snow precipitation and persistence (also increasing drought in the following summer) [77]. Araucaria forests have a long history of anthropogenic disturbances, including an intensive reduction in forest area since the arrival of Euro-American settlers through intentional fires for clearing lands for grazing [78•, 79] and intensive logging [80]. Most of the Araucaria forests were protected in the 1900s to ameliorate this reduction; however, the disturbance has continued in parts of their distribution. Overgrazing and cattle ranching [81], the introduction of alien mammals [82] and the increasing establishment of plantation forests have further contributed to the fragmentation and alteration of fire regimes [83, 84]. These disturbances have been shown to impact the health and regeneration capacity of the species. However, no large-scale studies have examined the link between these factors and tree health status. Consequently, the potential importance of these factors as drivers of this recently emerged decline remains unknown. Studying a new decline such as this across a large area and in rugged terrain is challenging. Below, we develop an experimental six-step approach to untangle the dynamics of complex tree declines.
Recommendations for an Experimental Approach to Studying Emerging Native Diseases
Here, we detail the steps of an experimental approach (Fig. 5), with an outline to the approach rather than describing all the possible factors to be explored, as we believe the outcome of each progressive step will guide the next step. We encourage the inclusion of multidisciplinary teams, encompassing at least forest pathologists, landscape ecologists, plant physiologists, soil scientists, climate modellers and biostatisticians as deemed necessary, as efforts to understand and mitigate these declines require a detailed understanding of multiple factors and potential mitigation options.
-
Step 1. Is a single pathogen consistently associated with the symptoms, or is there a more complex decline without a specific pathogen as a key component?
Systematic surveys of symptomatic and asymptomatic trees should be undertaken at multiple locations to develop an understanding of the disease aetiology, including disease progression, and if a primary pathogen/s can be consistently observed or not. The inclusion of specialists on different pathogens would increase the likelihood of correctly diagnosing the disease-causing agent. The possibility of involvement of multiple pathogens, the simultaneous emergence of disease syndromes and/or a general underlying decline deserves careful consideration at this initial stage.
In cases where a primary pathogen is found, answering questions regarding its origin and biology may become relevant, such as whether it is native or alien, or whether it has arisen through an evolutionary process (hosts jump, host shift or host range expansion). Suppose a diversity of secondary pathogens are detected. In that case, a stronger emphasis should turn towards the host’s condition, looking at whether there are any evident localised abiotic drivers or stressors, or if a broader range complex condition may be underlying, i.e. Manion decline spiral.
-
Step 2. Is this a local or large-scale problem?
Systematic surveys should be undertaken at a larger scale (ideally across the entire host range) to understand the scale of the issue. For example, is it occurring in a single stand, multiple stands or across the entire distribution range of the host? Assessing the consistency of symptoms and pathogens needs to be considered by applying standardised and coordinated methods for disease characterisation, scoring and sampling. Hyperspectral imaging and other remote sensing tools could be considered [85]. Fungal population genetics studies can be used to assess if genotypes associated with disease differ from those in asymptomatic trees [86] or if new genotypes have been introduced to a region [87], well-planed hierarchical sampling can also determine if there is structure within populations [88]. Numerous population genetics studies have been undertaken considering invasive pathogens [15], but there are few examples with native pathogens.
The presence of the pathogen across the range of the host and its isolation from asymptomatic trees provide strong evidence for it being native rather than alien; this is an important distinction as if it is an introduced alien pathogen, appropriate management goals should be determined, e.g. assessment of eradication feasibility, development of containment and disease mitigation strategies [7, 89]. For native pathogens, the expectation is that they will have a ubiquitous distribution, and management must focus on the release of the stressors driving the pathogen emergence.
-
Step 3. Is the disease associated with land-use change or human disturbance?
For this purpose, landscape-level studies of disease extent and distribution are required. Variables at this level include those related to human disturbance, e.g. distance to roads and hiking trails, changes in forest management (e.g. changes in logging regimes), and air and water pollution and other plot variables (e.g. average age of individuals per plot, canopy cover, sunlight penetration, slope, aspect, pH, soil moisture). These variables can be used in linear modelling approaches to see which are significant in predicting the health parameters of the populations. Surveys can be conducted to determine disease incidence in sites experiencing these disturbances or not (i.e. comparative study design). Surveys can include comparing damage inside/outside protected areas, comparing areas with contrasting management histories (e. g. wood extraction, grazing, others; policy changes) and comparing sites where restoration programmes may have been implemented (e. g. planting, removing alien vegetation and animals). Alternatively, a historical reconstruction of the forests (30–50–80 or longer years ago) can be included, combining information such as land-use changes, forest adaptation to human perturbation events or regimes still present or interrupted, and/or changes in forests management.
-
Step 4. Have there been changes in short- and/or long-term climate and/or weather conditions?
Climatic conditions can be considered at different temporal and spatial scales. Weather changes refer to short-term atmospheric conditions, while climate is the weather of a specific region averaged over a long time. Historical (long-term) climate data for sites showing different levels of disease/damage can provide evidence for correlation with trends in changing conditions such as changes in temperature (at different seasons) and precipitation (e. g. amount, time and frequency of occurrence, type, persistence). Both long- and short-term changes can result in a disease outbreak. Short-term weather data can be obtained in diverse manners (weather stations, on-site temperature/humidity recording devices such as iButtons) and combined with data (e.g. plant composition, growth rate, tree health, ground cover, litter depth) from long-term monitoring plots. Any changes in weather at either a local or landscape scale could then be related to changes found in the type and amount of damage present. Plant-based indicators are an alternative way to explore the change in climatic conditions and/or effect of such changes. These include, for example, studies of tree rings (which can link tree declines to changes in atmospheric conditions) or taking leaf samples and using C isotopic signature (δ13C) to obtain information regarding water scarcity.
-
Step 5. Have there been changes in other abiotic factors?
There are numerous additional abiotic factors, many of which may be linked to the anthropogenic changes observed in step 3, which may contribute to the incidence and severity of a disease outbreak. These include changes to soil structure due to compaction or other factors, changes in fire regimes, increased nutrients or imbalanced nutrients due to fertiliser use, pesticide and herbicide damage, changes in soil pH, increased salinity or altered drainage or water tables due to land-use management. These geophysical and hydrophysical changes will predominantly affect the host physiology and may reduce the hosts’ disease resistance.
-
Step 6. Have there been changes in other biotic factors?
As with step 5, numerous potential biotic interactions may influence a disease outbreak, and again many of these will be linked to anthropogenic disturbance: changes in vegetation (stand structure, abundance, composition, density, basal area age) due to edge effects, invasion by alien plant species, other pests and diseases, rhizosphere biota changes and mycorrhiza. These other biotic impacts could also be the result of climate, weather, land use, human disturbance and management and belowground changes and would indicate a complex interactive scenario, for example, rhizosphere-plant-pathogen interactions.
Changes in belowground microbial composition can result from changes in soil condition and plant communities, which, in turn, can be affected by human disturbances and changes in management. The most complex and challenging aspect of understanding tree declines with complex aetiologies is attempting to unravel these relationships.
Conclusion
Due to global change, more trees will exhibit a wide range of symptoms indicative of poor vigour, particularly in stressed species towards the limit of their native ranges and stands growing on marginal sites. By looking at four paradigmatic examples of forest decline, we found forest management had a significant role as predisposing and inciting factors for tree health. Land-use change is one of the three components of global change, including both climate change and globalisation, and more attention should be placed on its role on tree health. The prevalence of diseases caused by native pathogens with complicated aetiologies will increase, and interdisciplinary teams are needed to untangle the multiple biotic and abiotic causative factors and their interactions to reveal the primary drivers of the decline. Recognition, characterisation and management of these epidemics are challenging as native pathogens are often already widespread, and eradication is not viable. The next century will likely see an unprecedented rise in forest pathogen epidemics, both native and alien; there is a need for a predictive rather than a reactive response. Rather than focusing on ‘single pathogen-single host’ disease dynamics, a ‘whole ecosystem’ approach incorporates both biotic and abiotic aspects of a forest community and the multiple interactions amongst host, pathogen, mutualists, the environment and humans. A ‘whole ecosystem’ approach highlights the many aspects and entanglements of forest declines and enables us to respond with management options appropriate to each scenario. The framework proposed here provides a logical six-step approach to discovering the direct and indirect environmental drivers of a forest decline syndrome.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Ramsfield TD, Bentz BJ, Faccoli M, Jactel H, Brockerhoff EG. Forest health in a changing world: effects of globalization and climate change on forest insect and pathogen impacts. Forestry. 2016;89:245–52.
Agrios GN. Plant Pathology. Fifth ed. Elsevier Academic Press, 2005.
Burgess TI, Wingfield MJ. Impact of fungi in natural forest ecosystems; a focus on Eucalyptus. In: Sivasithamparam K, Dixon KW, Barrett RL, editors. Microorganisms in plant conservation and biodiversity. Dordrecht: Kluwer Academic Publishers; 2002. p. 285–306.
Burdon JJ, Thrall PH. Coevolution of plants and their pathogens in natural habitats. Science. 2009;324:755–6.
Stenlid J, Oliva J. Phenotypic interactions between tree hosts and invasive forest pathogens in the light of globalization and climate change. Philos Trans R Soc B Biol Sci. 2016;371:20150455.
Anderson PK, Cunningham AA, Patel NG, Morales FJ, Epstein PR, Daszak P. Emerging infectious diseases of plants: pathogen pollution, climate change and agrotechnology drivers. Trends Ecol Evol. 2004;19:535–44.
Paap T, Wingfield MJ, Burgess TI, Wilson JRU, Richardson DM, Santini A. Invasion frameworks: a forest pathogen perspective. Curr For Rep. 2022; 8:74–89
Bonnamour A, Gippet JMW, Bertelsmeier C. Insect and plant invasions follow two waves of globalisation. Ecol Lett. 2021. https://doi.org/10.1111/ele.13863.
Rizzo DM, Garbelotto M. Sudden oak death: endangering California and Oregon forest ecosystems. Front Ecol Environ. 2003;1:197–204.
Rigling D, Prospero S. Cryphonectria parasitica, the causal agent of chestnut blight: invasion history, population biology and disease control. Mol Plant Pathol. 2018;19:7–20.
Shearer BL, Crane CE, Barrett S, Cochrane A. Phytophthora cinnamomi invasion, a major threatening process to conservation of flora diversity in the South-west Botanical Province of Western Australia. Aust J Bot. 2007;55:225–38.
Brasier CM, Buck KW. Rapid evolutionary changes in a globally invading fungal pathogen (Dutch elm disease). Biol Invasions. 2001;3:223–33.
Pautasso M, Aas G, Queloz V, Holdenrieder O. European ash (Fraxinus excelsior) dieback–a conservation biology challenge. Biol Cons. 2013;158:37–49.
•• Desprez-Loustau M-L, Aguayo J, Dutech C, Hayden KJ, Husson C, Jakushkin B, et al. An evolutionary ecology perspective to address forest pathology challenges of today and tomorrow. Ann For Sci. 2016;73:45–67. An important review highlighting the need to consider evolutionary ecology perspectives in forest health research. The authors also provide policy recommendations and identify areas of research need.
Burgess TI, Wingfield MJ. Pathogens on the move: a 100 year global experiment with planted eucalypts. Bioscience. 2017;67:14–25.
•• Ghelardini L, Pepori AL, Luchi N, Capretti P, Santini A. Drivers of emerging fungal diseases of forest trees. For Ecol Manag. 2016;381:235–46. Provides an overview of host-pathogen-environment relationships with emphasis on interactions among anthropogenic disturbance, new and emerging fungi (i.e. invasive species, new strains, new hybrids and latent pathogens) and potential range shifts of fungi.
Hepting GH. Climate and forest diseases. Annu Rev Phytopathol. 1963;1:31–50.
Hennon PE, Frankel SJ, Woods AJ, Worrall JJ, Norlander D, Zambino PJ, et al. A framework to evaluate climate effects on forest tree diseases. For Pathol. 2020;50:e12649.
Sinclair WA. Comparisons of recent declines of white ash, oaks, and sugar maple in northeastern woodlands. Cornell Plantations. 1965;20:62–7.
Manion PD. Tree disease concepts. New Jersey: Prentice-Hall; 1981.
Manion PD. Tree disease concepts. Englewood Cliffs: Prentice Hall; 1991.
Holdenrieder O, Pautasso M, Weiberg PJ, Lonsdale D. Tree diseases and landscape processes: the challenge of landscape pathology. Trends Ecol Evol. 2004;19:446–52.
Desprez-Loustau M-L, Marçais B, Nageleisen LM, Piou D, Vannini A. Interactive effects of drought and pathogens in forest trees. Ann For Sci. 2006;63:597–612.
Paap T, Burgess TI, McComb JA, Shearer BL, Hardy GES. Quambalaria species, including Q. coyrecup sp. nov., implicated in canker and shoot blight diseases causing decline of Corymbia species in the southwest of Western Australia. Mycol Res. 2008;112:57–69.
Paap T, Burgess TI, Calver M, McComb J, Shearer B, Hardy GES. A thirteen-year study on the progression of a severe canker disease on the health of a keystone tree in a Mediterranean forest and woodland ecosystem. For Pathol. 2017;47:e12292.
Ramalho CE, Laliberté E, Poot P, Hobbs RJ. Complex effects of fragmentation on remnant woodland plant communities of a rapidly urbanizing biodiversity hotspot. Ecology. 2014;95:2466–78.
Zambrano J, Garzon-Lopez CX, Yeager L, Fortunel C, Cordeiro NJ, Beckman NG. The effects of habitat loss and fragmentation on plant functional traits and functional diversity: what do we know so far? Oecologia. 2019;191:505–18.
Arroyo-Rodríguez V, Saldana-Vazquez RA, Fahrig L, Santos BA. Does forest fragmentation cause an increase in forest temperature? Ecol Res. 2017;32:81–8.
Latimer CE, Zuckerberg B. Forest fragmentation alters winter microclimates and microrefugia in human-modified landscapes. Ecography. 2017;40:158–70.
Watson JEM, Evans T, Venter O, Williams B, Tulloch A, Stewart C, et al. The exceptional value of intact forest ecosystems. Nat Ecol Evol. 2018;2:599–610.
•• Paap T, Burgess TI, Rolo V, Steel E, Hardy GESJ. Anthropogenic disturbance impacts stand structure and susceptibility of an iconic tree species to an endemic canker pathogen. For Ecol Manag. 2018;425:145–53. Data driven paper based on extensive surveys showing a clear evidence for anthropogenic disturbance driving a tree decline.
Dawes W, Ali R, Varma S, Emelyanova I, Hodgson GA, McFarlane DJ. Modelling the effects of climate and land cover change on groundwater recharge in southwest Western Australia. Hydrol Earth Syst Sci. 2012;16:2709–22.
Matusick G, Ruthrof KX, Kala J, Brouwers NC, Breshears DD, Hardy GES. Chronic historical drought legacy exacerbates tree mortality and crown dieback during acute heatwave-compounded drought. Environ Res Lett. 2018;13:095002.
Ruthrof KX, Breshears DD, Fontaine JB, Froend RH, Matusick G, Kala J, et al. Subcontinental heat wave triggers terrestrial and marine, multi-taxa responses. Sci Rep. 2018;8:1–9.
• Sapsford SJ, Paap T, Hardy GESJ, Burgess TI. Anthropogenic disturbance impacts mycorrhizal communities and abiotic soil properties: implications for an endemic forest disease. Front For Glob Chang. 2021;3:e593243. Strong empirical evidence for the complex interactions between tree decline and soil biota.
Guo Q, Riitters KH, Potter KM. A subcontinental analysis of forest fragmentation effects on insect and disease invasion. Forests. 2018;9:744.
Burgess TI, Scott JK, McDougall KL, Stukely MJC, Crane C, Dunstan WA, et al. Current and projected global distribution of Phytophthora cinnamomi, one of the world’s worst plant pathogens. Glob Change Biol. 2017;23:1661–74.
Paap T, Brouwers N, Burgess TI, Hardy GES. Importance of climate, anthropogenic disturbance and pathogens (Quambalaria coyrecup and Phytophthora spp.) on marri (Corymbia calophylla) tree health in southwest Western Australia. Ann For Sci. 2017;74:62.
Croeser L, Admiraal R, Barber PA, Burgess TI, Hardy GESJ. Reflectance spectroscopy to characterise the response of Corymbia calophylla to Phytophthora root rot and waterlogging stress. Forestry. 2021. https://doi.org/10.1093/forestry/cpab045.
Corcobado T, Vivas M, Moreno G, Solla A. Ectomycorrhizal symbiosis in declining and non-declining Quercus ilex trees infected with or free of Phytophthora cinnamomi. For Ecol Manage. 2014;324:72–80.
Ennos RA. Resilience of forests to pathogens: an evolutionary ecology perspective. Forestry. 2015;88:41–52.
Hossain M, Veneklaas EJ, Hardy GESJ, Poot P. Tree host–pathogen interactions as influenced by drought timing: linking physiological performance, biochemical defence and disease severity. Tree Physiol. 2019;39:6–18.
Camarero JJ, Bigler C, Linares JC, Gil-Pelegrín E. Synergistic effects of past historical logging and drought on the decline of Pyrenean silver fir forests. For Ecol Manage. 2011;262:759–69.
Oliva J, Colinas C. Canopy openings may prevent fir broom rust (Melampsorella caryophyllacearum) infections. Eur J For Res. 2007;126:507–11.
•• Oliva J, Colinas C. Decline of silver fir (Abies alba Mill.) stands in the Spanish Pyrenees: role of management, historic dynamics and pathogens. For Ecol Manag. 2007;252:84–97. Good example of how to try to understand forest decline at a large scale by sampling stands at random and trying to link poor health with different pathogens.
Johannesson H, Stenlid J. Molecular identification of wood-inhabiting fungi in an unmanaged Picea abies forest in Sweden. For Ecol Manage. 1999;115:203–11.
Oliva J, Colinas C. Epidemiology of Heterobasidion abietinum and Viscum album on silver fir (Abies alba) stands of the Pyrenees. For Pathol. 2010;40:19–32.
Oliva J, Suz LM, Colinas C. Ecology of Armillaria species on silver fir (Abies alba) in the Spanish Pyrenees. Ann For Sci. 2009;66:603.
•• Sangüesa-Barreda G, Camarero JJ, Oliva J, Montes F, Gazol A. Past logging, drought and pathogens interact and contribute to forest dieback. Agric For Meteorol. 2015;208:85–94. Shows how declining trees were mostly dominant or co-dominant trees that were released after selective cuttings.
Améztegui A, Brotons L, Coll L. Land-use changes as major drivers of mountain pine (Pinus uncinata Ram) expansion in the Pyrenees. Glob Ecol Biogeogr. 2010;19:632–41.
Slippers B, Hurley BP, Wingfield MJ. Sirex woodwasp: a model for evolving management paradigms of invasive forest pests. Annu Rev Entomol. 2015;60:601–19.
Camarero JJ, Gazol A, Sangüesa-Barreda G, Oliva J, Vicente-Serrano SM. To die or not to die: early warnings of tree dieback in response to a severe drought. J Ecol. 2015;103:44–57.
Macias M, Andreu L, Bosch O, Camarero JJ, Gutiérrez E. Increasing aridity is enhancing silver fir (Abies alba mill.) water stress in its south-western distribution limit. Clim Chang. 2006;79:289–313.
Gazol A, Camarero JJ, Gutiérrez E, Popa I, Andreu-Hayles L, Motta R, et al. Distinct effects of climate warming on populations of silver fir (Abies alba) across Europe. J Biogeogr. 2015;42:1150–62.
Marqués L, Peltier DMP, Camarero JJ, MlA Zavala, Madrigal-González J, Sangüesa-Barreda G, et al. Disentangling the legacies of climate and management on tree growth. Ecosystems. 2021. https://doi.org/10.1007/s10021-021-00650-8.
Peck C. Annual report on the New York State Museum of Natural History. Report of the botanist. Albany, Regents of the University of the State of New York. 1980. Available at: https://www.biodiversitylibrary.org/item/110212.
Fitzpatrick HM. Monograph of the Coryneliaceae. Mycologia. 1920;12:206–37.
•• Munck IA, Livingston WH, Lombard K, Luther T, Ostrofsky WD, Weimer J, et al. Extent and severity of Caliciopsis canker in New England, USA: an emerging disease of eastern white pine (Pinus strobus L.). Forests. 2015;6:4360–73. One of the first papers describing the extent of Caliciopsis canker in New England and linking disease incidence with tree size, stand density and soil type.
•• Schulz AN, Mech AM, Asaro C, Coyle DR, Cram MM, Lucardi RD, et al. Assessment of abiotic and biotic factors associated with eastern white pine (Pinus strobus L.) dieback in the Southern Appalachian Mountains. For Ecol Manag. 2018;423:59–69. This paper considers numerous biotic and abiotic factors driving the landscape level impacts of white pine dieback.
Livingston WH, Kenefic LS. Low densities in white pine stands reduce risk of drought-incited decline. For Ecol Manage. 2018;423:84–93.
Costanza KKL, Whitney TD, McIntire CD, Livingston WH, Gandhi KJK. A synthesis of emerging health issues of eastern white pine (Pinus strobus) in eastern North America. For Ecol Manage. 2018;423:3–17.
Costanza KKL, Crandall MS, Rice RW, Livingston WH, Munck IA, Lombard K. Economic implications of a native tree disease, Caliciopsis canker, on the white pine (Pinus strobus) lumber industry in the northeastern United States. Can J For Res. 2019;49:521–30.
Hayhoe K, Wake CP, Huntington TG, Luo L, Schwartz MD, Sheffield J, et al. Past and future changes in climate and hydrological indicators in the US Northeast. Clim Dyn. 2007;28:381–407.
Hayhoe K, VanDorn J, Croley T II, Schlegal N, Wuebbles D. Regional climate change projections for Chicago and the US Great Lakes. J Great Lakes Res. 2010;36:7–21.
•• Simler-Williamson AB, Rizzo DM, Cobb RC. Interacting effects of global change on forest pest and pathogen dynamics. Ann Rev Ecol Evol Syst. 2019;50:381–403. Review paper summarizing the interactions between tree mortality, pathogens, pests, the environment and climate change. The authors develop a framework to explain how diverse global change drivers impact the disease triangle.
Wyka SA, Smith C, Munck IA, Rock BN, Ziniti BL, Broders K. Emergence of white pine needle damage in the northeastern United States is associated with changes in pathogen pressure in response to climate change. Glob Change Biol. 2017;23:394–405.
Munck IA, Luther T, Wyka S, Keirstead D, McCracken K, Ostrofsky WD, et al. Soil and stocking effects on Caliciopsis canker of Pinus strobus L. Forests. 2016;7:269.
• Costanza KKL, Livingston WH, Fraver S, Munck IA. Dendrochronological analyses and whole-tree dissections reveal Caliciopsis canker (Caliciopsis pinea) damage associated with the declining growth and climatic stressors of eastern White Pine (Pinus strobus). Forests. 2020;11:347. Paper that used dendrochronology and whole-tree dissection to link a decrease in tree growth to tree damage, i.e. cankers with abiotic stressors (weather events—droughts and a hurricane).
Whitney TD, Cram MM, Barnes BF, Yao J, Lucardi RD, Gandhi KJK. Tree-level distribution of a novel insect-pathogen complex and its potential contribution to eastern white pine dieback. For Ecol Manage. 2018;423:49–58.
Mech AM, Asaro C, Cram MM, Coyle DR, Gullan PJ, Cook LG, et al. Matsucoccus macrocicatrices (Hemiptera: Matsucoccidae): first report, distribution, and association with symptomatic eastern white pine in the southeastern United States. J Econ Entomol. 2013;106:2391–8.
Broders K, Munck I, Wyka S, Iriarte G, Beaudoin E. Characterization of fungal pathogens associated with white pine needle damage (WPND) in northeastern North America. Forests. 2015;6:4088–104.
Livingston WH, Munck I, Lombard K, Weimer J, Bergdahl A, Kenefic LS et al. MP764: Field Manual for Managing Eastern White Pine Health in New England. Maine Agricultural and Forest Experiment Station Miscellaneous Publications. 2019. https://digitalcommons.library.umaine.edu/aes_miscpubs/24
Vélez ML, Marfetán JA, Salgado Salomon ME, Taccari LE. Mortierella species from declining Araucaria araucana trees in Patagonia Argentina. For Pathol. 2020;50:e12591.
Balocchi F, Wingfield MJ, Ahumada R, Barnes I. Pewenomyces kutranfy gen. nov. et sp. nov. causal agent of an important canker disease on Araucaria araucana in Chile. Plant Pathol. 2021;70:1243–59.
Vélez ML, Salgado Salomón ME, Marfetan A, Tirante SI, Mattes Fernández H, Avila M, Caracterización desecación del dosel y sanidad de Araucaria araucana en Argentina. Technical Report. 2018. Available from: https://doi.org/10.13140/RG.2.2.14227.78889.
Garreaud RD, Boisier JP, Rondanelli R, Montecinos A, Sepúlveda HH, Veloso-Aguila D. The central Chile mega drought (2010–2018): a climate dynamics perspective. Int J Climatol. 2020;40:421–39.
Cordero RR, Asencio V, Feron S, Damiani A, Llanillo PJ, Sepulveda E, et al. Dry-season snow cover losses in the Andes (18°–40° S) driven by changes in large-scale climate modes. Sci Rep. 2019;9:1–10.
• Moreno-Gonzalez R, Giesecke T, Fontana SL. The impact of recent land-use change in the Araucaria araucana forest in northern Patagonia. Holocene. 2020;30:1101–14. Holistic study of different types of impacts and how they effect a forest.
Mundo IA, Kitzberger T, Juñent FAR, Villalba R, Barrera MD. Fire history in the Araucaria araucana forests of Argentina: human and climate influences. Int J Wildland Fire. 2012;22:194–206.
Aagesen DL. Indigenous resource rights and conservation of the monkey-puzzle tree (Araucaria araucana, Araucariaceae): a case study from southern Chile. Econ Bot. 1998;52:146–60.
Zamorano-Elgueta C, Cayuela L, González-Espinosa M, Lara A, Parra-Vázquez MR. Impacts of cattle on the South American temperate forests: challenges for the conservation of the endangered monkey puzzle tree (Araucaria araucana) in Chile. Biol Cons. 2012;152:110–8.
Shepherd JD, Ditgen RS. Human use and small mammal communities of Araucaria forests in Neuquén, Argentina. Mastozoología Neotropical. 2005;12:217–26.
Cóbar-Carranza AJ, García RA, Pauchard A, Pena E. Effect of Pinus contorta invasion on forest fuel properties and its potential implications on the fire regime of Araucaria araucana and Nothofagus antarctica forests. Biol Invasions. 2014;16:2273–91.
Molina JR, Martin A, Drake F, Martín LM, Herrera MÁ. Fragmentation of Araucaria araucana forests in Chile: quantification and correlation with structural variables. iForest-Biogeosci For. 2015;9:244.
Dash JP, Watt MS, Pearse GD, Heaphy M, Dungey HS. Assessing very high resolution UAV imagery for monitoring forest health during a simulated disease outbreak. ISPRS J Photogramm Remote Sens. 2017;131:1–14.
Dakin N, White D, Hardy GES, Burgess TI. The opportunistic pathogen, Neofusicoccum australe, is responsible for crown dieback of peppermint (Agonis flexuosa) in Western Australia. Australas Plant Pathol. 2010;39:202–6.
Dutech C, Barrès B, Bridier J, Robin C, Milgroom MG, Ravigné V. The chestnut blight fungus world tour: successive introduction events from diverse origins in an invasive plant fungal pathogen. Mol Ecol. 2012;21:3931–46.
McDonald BA, Linde C. Pathogen population genetics, evolutionary potential and durable resistance. Annu Rev Phytopathol. 2002;40:349–79.
Paap T, Wingfield MJ, Burgess TI, Hulbert JM, Santini A. Harmonising the fields of invasion science and forest pathology. NeoBiota. 2020;62:301–32.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions JO was supported by the ‘Ramón y Cajal’ fellowship RYC-2015–17459 from the Ministry of Science and Education of Spain. USDA Forest Service Forest Health Protection partially supported MLS.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Forest Pathology
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Burgess, T.I., Oliva, J., Sapsford, S.J. et al. Anthropogenic Disturbances and the Emergence of Native Diseases: a Threat to Forest Health. Curr Forestry Rep 8, 111–123 (2022). https://doi.org/10.1007/s40725-022-00163-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40725-022-00163-0