Abstract
Introduction
This study aimed to meta-analyze the functional magnetic resonance imaging (fMRI) data and compare the brain activations from gustatory studies with different stimulus delivery methods.
Methods
Published fMRI studies were included into the analysis if they evaluated the brain responses to liquid tastants or food among healthy subjects. Studies were coded into three groups: stimulus removed from the mouth by suction without the need to swallow, swallowing without controlling for its confounds, and swallowing with controlling for its confounds.
Results
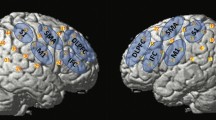
Forty-five studies that comprised of a total of 1498 participants fulfilled the inclusion criteria. Suction studies activated the posterior cingulate. Swallowing studies covered multiple brain regions including the anterior and middle insula, the precentral gyrus, and the postcentral gyrus.
Conclusions
For the contrast analysis between swallowing studies with and without controlling for the confounds, the former group had larger brain activation mainly at the anterior and middle insula and the thalamus, whereas the latter group had larger brain activation mainly at the anterior cingulate, precentral gyrus, and postcentral gyrus.
Implications
Compared to studies that did not control for confounds of swallowing, studies that controlled for swallowing demonstrated heightened responses at the insula and reduced responses at the sensorimotor cortex.


Similar content being viewed by others
References
Avery JA et al (2020) Taste quality representation in the human brain. J Neurosci 40:1042–1052
Bender G et al (2009) Neural correlates of evaluative compared with passive tasting. Eur J Neurosci 30:327–338
Birn RM et al (1999) Event-related fMRI of tasks involving brief motion. Hum Brain Mapp 7:106–114
Burger KS (2017) Frontostriatal and behavioral adaptations to daily sugar-sweetened beverage intake: a randomized controlled trial. Am J Clin Nutr 105:555–563
Burger KS, Stice E (2014) Neural responsivity during soft drink intake, anticipation, and advertisement exposure in habitually consuming youth. Obesity 22:441–450
Camps G et al (2018) Just add water: effects of added gastric distention by water on gastric emptying and satiety related brain activity. Appetite 127:195–202
Cerf B et al (1998) Functional lateralization of human gustatory cortex related to handedness disclosed by fMRI study. Ann N Y Acad Sci 855:575–578
Chen EY, Zeffiro TA (2020) Hunger and BMI modulate neural responses to sweet stimuli: fMRI meta-analysis. Int J Obes 44:1636–1652
Dalenberg JR et al (2017) Flavor pleasantness processing in the ventral emotion network. PLoS ONE 12:e0170310
Dalenberg JR et al (2018) Valence processing differs across stimulus modalities. Neuroimage 183:734–744
Dalenberg JR et al (2020) Short-term consumption of sucralose with, but not without, carbohydrate impairs neural and metabolic sensitivity to sugar in humans. Cell Metab 31(493–502):e7
De Araujo IE et al (2003) Taste-olfactory convergence, and the representation of the pleasantness of flavour, in the human brain. Eur J Neurosci 18:2059–2068
Ebisch SJ et al (2015) Emotional susceptibility trait modulates insula responses and functional connectivity in flavor processing. Front Behav Neurosci 9:297
Eickhoff SB et al (2009) Coordinate-based ALE meta-analysis of neuroimaging data: a random-effects approach based on empirical estimates of spatial uncertainty. Hum Brain Mapp 30:2907
Eickhoff SB et al (2012) Activation likelihood estimation meta-analysis revisited. Neuroimage 59:2349–2361
Eiler WJ II et al (2018) Family history of alcoholism and the human brain response to oral sucrose. NeuroImage Clinical 17:1036–1046
Faurion A et al (1999) Human taste cortical areas studied with functional magnetic resonance imaging: evidence of functional lateralization related to handedness. Neurosci Lett 277:189–192
Frost R et al (2015) What can the brain teach us about winemaking? An fMRI study of alcohol level preferences. PLoS ONE 10:e0119220
Goto TK et al (2016) Enhancement of combined umami and salty taste by glutathione in the human tongue and brain. Chem Senses 41:623–630
Grabenhorst F, Rolls ET, Bilderbeck A (2008) How cognition modulates affective responses to taste and flavor: top-down influences on the orbitofrontal and pregenual cingulate cortices. Cereb Cortex 18:1549–1559
Green BG, Nachtigal D (2012) Somatosensory factors in taste perception: effects of active tasting and solution temperature. Physiol Behav 107:488–495
Green E et al (2013) Can age-related CNS taste differences be detected as early as middle age? Evidence from fMRI. Neuroscience 232:194–203
Haase L et al (2007) On-line psychophysical data acquisition and event-related fMRI protocol optimized for the investigation of brain activation in response to gustatory stimuli. J Neurosci Methods 159:98–107
Haase L, Cerf-Ducastel B, Murphy C (2009) Cortical activation in response to pure taste stimuli during the physiological states of hunger and satiety. Neuroimage 44:1008–1021
Haase L, Green E, Murphy C (2011) Males and females show differential brain activation to taste when hungry and sated in gustatory and reward areas. Appetite 57:421–434
Han P et al (2018) Different neural processing of umami and salty taste determined by umami identification ability independent of repeated umami exposure. Neuroscience 383:74–83
Holmes CJ et al (1998) Enhancement of MR images using registration for signal averaging. J Comput Assist Tomogr 22:324–333
Huerta CI et al (2014) Neural bases of food perception: coordinate-based meta-analyses of neuroimaging studies in multiple modalities. Obesity 22:1439–1446
Iannilli E et al (2014) Oral texture influences the neural processing of ortho-and retronasal odors in humans. Brain Res 1587:77–87
Jacobson A, Green E, Murphy C (2010) Age-related functional changes in gustatory and reward processing regions: an fMRI study. Neuroimage 53:602–610
Kami YN et al (2008) The development of a novel automated taste stimulus delivery system for fMRI studies on the human cortical segregation of taste. J Neurosci Methods 172:48–53
Kawakami S et al (2016) The brain mechanisms underlying the perception of pungent taste of capsaicin and the subsequent autonomic responses. Front Hum Neurosci 9:720
Kobayashi M et al (2004) Functional imaging of gustatory perception and imagery: “top-down” processing of gustatory signals. Neuroimage 23:1271–1282
Lancaster JL et al (2007) Bias between MNI and Talairach coordinates analyzed using the ICBM-152 brain template. Hum Brain Mapp 28:1194–1205
McCabe C et al (2011) The D2 antagonist sulpiride modulates the neural processing of both rewarding and aversive stimuli in healthy volunteers. Psychopharmacology 217:271–278
McCabe C et al (2012) Neural processing of reward and punishment in young people at increased familial risk of depression. Biol Psychiat 72:588–594
McCabe C et al (2013) Effects of pramipexole on the processing of rewarding and aversive taste stimuli. Psychopharmacology 228:283–290
Müller VI et al (2018) Ten simple rules for neuroimaging meta-analysis. Neurosci Biobehav Rev 84:151–161
Murray E et al (2014) Opposing neural effects of naltrexone on food reward and aversion: implications for the treatment of obesity. Psychopharmacology 231:4323–4335
Nakamura Y et al (2011) Localization of brain activation by umami taste in humans. Brain Res 1406:18–29
Nakamura Y et al (2012) The temporal change in the cortical activations due to salty and sweet tastes in humans: fMRI and time–intensity sensory evaluation. NeuroReport 23:400–404
Nakamura Y et al (2013) Localization of the primary taste cortex by contrasting passive and attentive conditions. Exp Brain Res 227:185–197
Nakamura Y et al (2020) Difference in neural reactivity to taste stimuli and visual food stimuli in neural circuits of ingestive behavior. Brain Imaging Behav 14:1395–1405
Oberndorfer TA et al (2013) Altered insula response to sweet taste processing after recovery from anorexia and bulimia nervosa. Am J Psychiatry 170:1143–1151
Ogawa H et al (2005) Functional MRI detection of activation in the primary gustatory cortices in humans. Chem Senses 30:583–592
Pazart L et al (2014) An fMRI study on the influence of sommeliers’ expertise on the integration of flavor. Front Behav Neurosci 8:358
Research Imaging Institute, 2016. GingerALE Version 2.3.6. Vol. 2017, ed.^eds.
Research Imaging Institute, UTHSCSA, 2016. Mango for the Desktop. Vol. 2017, ed.^eds.
Roberts CA et al (2020) A systematic review and activation likelihood estimation meta-analysis of fMRI studies on sweet taste in humans. J Nutr 150:1619–1630
Rolls ET, McCabe C (2007) Enhanced affective brain representations of chocolate in cravers vs. non-cravers. Eur J Neurosci 26:1067–1076
Rolls ET, Kellerhals MB, Nichols TE (2015) Age differences in the brain mechanisms of good taste. Neuroimage 113:298–309
Rudenga K et al (2010) Evidence for an integrated oral sensory module in the human anterior ventral insula. Chem Senses 35:693–703
Sadler JR et al (2021) Correlates of neural adaptation to food cues and taste: the role of obesity risk factors. Social Cognitive and Affective Neuroscience. https://doi.org/10.1093/scan/nsab018
Small DM et al (2003) Dissociation of neural representation of intensity and affective valuation in human gustation. Neuron 39:701–711
Sörös P, Inamoto Y, Martin RE (2009) Functional brain imaging of swallowing: an activation likelihood estimation meta-analysis. Hum Brain Mapp 30:2426–2439
Spetter M et al (2010) Representation of sweet and salty taste intensity in the brain. Chem Senses 35:831–840
Spetter MS et al (2014) The sum of its parts—effects of gastric distention, nutrient content and sensory stimulation on brain activation. PLoS ONE 9:e90872
Stice E et al (2012) Multilocus genetic composite reflecting dopamine signaling capacity predicts reward circuitry responsivity. J Neurosci 32:10093–10100
Stice E, Burger KS, Yokum S (2013) Relative ability of fat and sugar tastes to activate reward, gustatory, and somatosensory regions. Am J Clin Nutr 98:1377–1384
Suen JLK et al (2021) Effective connectivity in the human brain for sour taste, retronasal smell, and combined flavour. Foods 10:2034
Takai O, Brown S, Liotti M (2010) Representation of the speech effectors in the human motor cortex: somatotopy or overlap? Brain Lang 113:39–44
Tord DM, et al., 2021b. 3D printed pacifier-shaped mouthpiece for fMRI-compatible gustometers. bioRxiv.
Tord DM, et al., 2021a. 3D printed pacifier-shaped mouthpiece for fMRI-compatible gustometers. eNeuro. 8, ENEURO.0208–21.
Tudge L, et al., 2015. Neural effects of cannabinoid CB1 neutral antagonist tetrahydrocannabivarin on food reward and aversion in healthy volunteers. International Journal of Neuropsychopharmacology. 18.
Turkeltaub PE et al (2012) Minimizing within-experiment and within-group effects in activation likelihood estimation meta-analyses. Hum Brain Mapp 33:1–13
van der Laan LN et al (2011) The first taste is always with the eyes: a meta-analysis on the neural correlates of processing visual food cues. Neuroimage 55:296–303
Veldhuizen MG, Small DM (2011) Modality-specific neural effects of selective attention to taste and odor. Chem Senses 36:747–760
Veldhuizen MG et al (2007) Trying to detect taste in a tasteless solution: modulation of early gustatory cortex by attention to taste. Chem Senses 32:569–581
Veldhuizen MG et al (2011) Identification of human gustatory cortex by activation likelihood estimation. Hum Brain Mapp 32:2256–2266
Veldhuizen MG, Gitelman DR, Small DM (2012) An fMRI study of the interactions between the attention and the gustatory networks. Chemosens Percept 5:117–127
Woods AT et al (2011) Expected taste intensity affects response to sweet drinks in primary taste cortex. NeuroReport 22:365–369
Yang X et al (2019) Negative affect amplifies the relation between appetitive-food-related neural responses and weight gain over three-year follow-up among adolescents. NeuroImage Clinical 24:102067
Yeung AWK (2018) Sex differences in brain responses to food stimuli: a meta-analysis on neuroimaging studies. Obes Rev 19:1110–1115
Yeung AWK (2021) Brain responses to watching food commercials compared with nonfood commercials: a meta-analysis on neuroimaging studies. Public Health Nutr 24:2153–2160
Yeung AWK, Goto TK, Leung WK (2017) Basic taste processing recruits bilateral anteroventral and middle dorsal insulae: an activation likelihood estimation meta-analysis of fMRI studies. Brain and Behavior 7:e00655
Yeung AWK, Goto TK, Leung WK (2018) Affective value, intensity and quality of liquid tastants/food discernment in the human brain: an activation likelihood estimation meta-analysis. Neuroimage 169:189–199
Yeung AWK, Wong NSM, Eickhoff SB (2020) Empirical assessment of changing sample-characteristics in task-fMRI over two decades: an example from gustatory and food studies. Hum Brain Mapp 41:2460–2473
Yeung AWK, Wong NSM, 2021. The non-transparent usage and reporting of the Edinburgh Handedness Inventory in functional magnetic resonance imaging literature: a survey of studies published since 2013. Laterality. [Epub ahead of print], https://doi.org/10.1080/1357650X.2021.1984497.
Funding
This work was supported by departmental funds only.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The author declares no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yeung, A.W.K. Differences in Brain Responses to Food or Tastants Delivered with and Without Swallowing: a Meta-analysis on Functional Magnetic Resonance Imaging (fMRI) Studies. Chem. Percept. 15, 112–123 (2022). https://doi.org/10.1007/s12078-022-09299-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12078-022-09299-6