Abstract
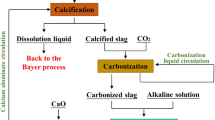
The calcification–carbonization method can effectively treat bauxite residue. In this paper, carbide slag is used as the calcium source of the calcification process to treat bauxite residue, which greatly reduces the process cost while realizing the utilization of two kinds of solid waste resources. Through the investigation of the influencing factors of the calcification process, we ascertained the optimal calcification condition is the calcium-to-silicon ratio of 2.5, calcification temperature of 160°C, the liquid-to-solid ratio of 5 : 1, and reaction duration of 60 min. Under this condition, a Na2O recovery rate of 94.7% was achieved, and the extraction rates of Al2O3 reach 33.9%. The main phase composition of tailings after treatment is CaCO3 and CaSiO4, which are environmentally harmless and can be reused as raw materials. On the other hand, using carbide slag to treat 1t bauxite residue can save 15.69$ of production cost, and the comprehensive economic benefit can reach 26.67$ per ton. Therefore, carbide slag is promising as a calcium source in the treatment of bauxite residue.












Similar content being viewed by others
REFERENCES
Shao wei, Y., Yi, Z., et al., Transformation of NaCaHSiO4 to sodalite and katoite in sodium aluminate solution, Hydrometallurgy, 2014, vol. 141, pp. 43–48. https://doi.org/10.1016/j.hydromet.2013.10.004.
Borges, A.J.P., Hauser-Davis, R.A., and de Oliveira, T.F., Cleaner red mud residue production at an alumina plant by applying experimental design techniques in the filtration stage, J. Cleaner Prod., 2011, vol. 19, pp. 1763–1769. https://doi.org/10.1016/j.jclepro.2011.06.006
Brunori, C., Cremisini, C., Massanisso, P., et al., Reuse of a treated red mud bauxite waste: studies on environmental compatibility, J. Hazard. Mater., 2005, vol. 117, no. 1, pp. 55–63. https://doi.org/10.1016/j.jhazmat.2004.09.010
Ghosh, I., Guha, S., Balasubramaniam, R., et al., Leaching of metals from fresh and sintered red mud, J. Hazard. Mater., 2011, vol. 185, nos. 2–3, pp. 662–668. https://doi.org/10.1016/j.jhazmat.2010.09.069
Wan chao, L., Yang, J., and Xiao, B., Application of Bayer red mud for iron recovery and building material production from alumosilicate residues, J. Hazard. Mater., 2009, vol. 161, pp. 474–478. https://doi.org/10.1016/j.jhazmat.2008.03.122
Édith, P., Blais, J.F., and Mercier, G., Transformation of red mud from aluminium industry into a coagulant for wastewater treatment, Hydrometallurgy, 2008, vol. 92, nos. 1–2, pp. 16–25. https://doi.org/10.1016/j.hydromet.2008.02.004
Palmer, S.J., Reddy, B.J., and Frost, R.L., Characterisation of red mud by UV-vis-NIR spectroscopy, Spectrochim. Acta, Part A, 2009, vol. 71, no. 5, pp. 1814–1818. https://doi.org/10.1016/j.saa.2008.06.038
Guo zhi, L. Ting an, Z., Chao zhen, Z., Xiao feng, Z., Wei guang, Z., and Yan xiu, W., The influence of the silicon saturation coefficient on a calcification carbonation method for clean and efficient use of bauxite, Hydrometallurgy, 2017, vol. 174, pp. 97–104. https://doi.org/10.1016/j.hydromet.2017.07.001
Guo zhi, L., Ting an, Z., Xiao feng, Z., et al., Calcification-carbonation method for cleaner alumina production and CO2 utilization, JOM, 2014, vol. 66, no. 9, pp. 1616–1621. https://doi.org/10.1007/s11837-014-1090-0
Power, G., Gräfe, M., and Klauber, C., Bauxite residue issues: I. Current management, disposal and storage practices, Hydrometallurgy, 2011, vol. 108, nos. 1–2, pp. 33–45. https://doi.org/10.1016/j.hydromet.2011.02.006
Tang, W.C., Wang, Z., Liu, Y., et al., Influence of red mud on fresh and hardened properties of self-compacting concrete, Constr. Build. Mater., 2018, vol. 178, pp. 288–300. https://doi.org/10.1016/j.conbuildmat.2018.05.171
Ri bin, L., Ting an, Z., Yan, L., et al., Calcification-carbonation method for red mud processing, J. Hazard. Mater., 2016, vol. 316, no. 5, pp. 94–101. https://doi.org/10.1016/j.jhazmat.2016.04.072.
Wan chao, L., et al., Environmental assessment, management and utilization of red mud in China, J. Cleaner Prod., 2014, vol. 84, pp. 606–610. https://doi.org/10.1016/j.jclepro.2014.06.080
Xuan, D., Zhan, B., Poon, C.S., et al., Innovative reuse of concrete slurry waste from ready-mixed concrete plants in construction products, J. Hazard. Mater., 2016, vol. 312, pp. 65–72. https://doi.org/10.1016/j.jhazmat.2016.03.036
Li, L.Y., A study of iron mineral transformation to reduce red mud tailings, Waste Manage., 2001, vol. 21, no. 6, pp. 525–534. https://doi.org/10.1016/S0956-053X(00)00107-0
Wan chao, L., Shou yi, S., and Ling, Z., Experimental and simulative study on phase transformation in Bayer red mud soda-lime roasting system and recovery of Al, Na and Fe, Miner. Eng., 2012, vol. 39, pp. 213–218. https://doi.org/10.1016/j.mineng.2012.05.021
Gelencsér, A., Kováts, N., Turóoczi, B., Rostási, Á., Hoffer, A., Imre, K., Nyirő-Kósa, I., Csákberényi-Malasics, D., Tóth, Á., Czitrovsky, A., Nagy, A., Nagy, S., Ács, A., Kovács, A., Ferincz, Á., Hartyáni, Z., and Pósfai, M., The red mud accident in Ajka (Hungary): Characterization and potential health effects of fugitive dust, Environ. Sci. Technol., 2011, vol. 45, pp. 1608–1615. https://doi.org/10.1021/es104005r
Zhu, L.P., Ni, W., and Zhang, X.F., Experimental study on preparation of total tailings cementing filling material by red mud-slag less clinker system, Metal Mine, 2009, vol. 39, no. 11, pp. 175–178. CNKI: SUN:JSKS.0.2009-11-053
Fang hai, L., et al., Recovery of gallium from Bayer red mud through acidic-leaching-ion-exchange process under normal atmospheric pressure, Hydrometallurgy, 2018, vol. 175, pp. 124–132. https://doi.org/10.1016/j.hydromet.2017.10.032.
Da wei, L., et al., Properties and mechanism of red mud in preparation of ultra-lightweight sludge-red mud ceramics, J. Cent. South Univ., 2012, vol. 19, pp. 231–237. https://doi.org/10.1007/s11771-012-0996-3
Li, H.X. and Liu, S., Metallurgical process for valuable elements recovery from red mud—A review, Hydrometallurgy, 2015, vol. 155, pp. 29–34. https://doi.org/10.1016/j.hydromet.2015.03.018
Xiao bin, L., et al., Reaction behaviors of iron and hematite in sodium aluminate solution at elevated temperature, Hydrometallurgy, 2017, vol. 175, pp. 257–265. https://doi.org/10.1016/j.hydromet.2017.12.004.
Samal, S., Ray, A.K., and Bandopadhyay, A., Characterization and microstructure observation of sintered red mud-fly ash mixtures at various elevated temperature, J. Cleaner Prod., 2015, vol. 101, pp. 368–376. https://doi.org/10.1016/j.jclepro.2015.04.010
Chun fa, L., Hui ming, L., Ding fan, Q., et al., Recovering valuable metals from red mud generation during alumina production, Light Met., 2003, no. 10, pp. 18–22.
Bai, J.W. and Deng, Y.Q., Analysis of mud-slag of calcium carbide, Phys. Test. Chem. Anal., Part B, 2004, vol. 40, no. 11, pp. 659–658. https://doi.org/10.1038/sj.cr.7290254
Ying jie, L., et al., CO2 capture performance of synthetic sorbent prepared from carbide slag and aluminum nitrate hydrate by combustion synthesis, Appl. Energy, 2015, vol. 145, pp. 60–68. https://doi.org/10.1016/j.apenergy.2015.01.061
Hui, Z., Xiang pin, L., and Jian ming, O.Y., Effects of supersaturation, stoichiometric ratio of reactants and ionic strength on the growth of calcium oxalate crystals, J. Inorg. Chem., 2005, vol. 21, no. 009, pp. 1375–1378. https://doi.org/10.3321/j.issn:1001-4861.2005.09.020
Verbeck, G., Carbonation of hydrated Portland cement, Portland Cem. Assoc., Res. Dev. Lab. Bull., 1958, no. 205, p. 20.
Hu, Z.S., Shao, M.H., Cai, Q., et al., Synthesis of needle-like aragonite from limestone in the presence of magnesium chloride, J. Mater. Process. Technol., 2009, vol. 209, no. 3, pp. 1607–1611. https://doi.org/10.1016/j.jmatprotec.2008.04.008
ACKNOWLEDGMENTS
This work was financially supported by the National Natural Science Foundation of China (nos. 51874078, U1710257, U1202274), the National key research and development plan (2017YFC0210403-04), the Fundamental Research Funds for the Central Universities of China (N2025038).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest.
About this article
Cite this article
Chen, Y., Lv, G., Zhang, Ta. et al. Carbide Slag as a Calcium Source for Bauxite Residue Utilization via Calcification–Carbonization Processing. Russ. J. Non-ferrous Metals 63, 132–145 (2022). https://doi.org/10.3103/S1067821222020043
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.3103/S1067821222020043