Abstract
The hygiene hypothesis has been popularized as an explanation for the rapid increase in allergic disease observed over the past 50 years. Subsequent epidemiological studies have described the protective effects that in utero and early life exposures to an environment high in microbial diversity have in conferring protective benefits against the development of allergic diseases. The rapid advancement in next generation sequencing technology has allowed for analysis of the diverse nature of microbial communities present in the barrier organs and a determination of their role in the induction of allergic disease. Here, we discuss the recent literature describing how colonization of barrier organs during early life by the microbiota influences the development of the adaptive immune system. In parallel, mechanistic studies have delivered insight into the pathogenesis of disease, by demonstrating the comparative effects of protective T regulatory (Treg) cells, with inflammatory T helper 2 (Th2) cells in the development of immune tolerance or induction of an allergic response. More recently, a significant advancement in our understanding into how interactions between the adaptive immune system and microbially derived factors play a central role in the development of allergic disease has emerged. Providing a deeper understanding of the symbiotic relationship between our microbiome and immune system, which explains key observations made by the hygiene hypothesis. By studying how perturbations that drive dysbiosis of the microbiome can cause allergic disease, we stand to benefit by delineating the protective versus pathogenic aspects of human interactions with our microbial companions, allowing us to better harness the use of microbial agents in the design of novel prophylactic and therapeutic strategies.
Similar content being viewed by others
Introduction
Atopic diseases such as asthma, hay fever, atopic dermatitis, and food allergies represent the most common forms of allergy and are typically defined by the presence of specific immunoglobulin E (sIgE) in serum or a positive skin prick test for common environmental allergens. Constituting the most prevalent chronic condition of childhood, significant proportions of children develop atopic symptoms in their first year of life. One recent multinational study indicated that 14–28% of infants suffer from atopic dermatitis [1] and rates of recurrent, severe wheezing often used as an early diagnostic marker of heightened risk for the development of asthma have been reported at 16% [2], with some western countries reporting rates of food allergy in excess of 10% at 12 months of age [3]. Increases in the prevalence of these conditions have largely been observed in industrialized countries and have been linked to the modern western diet and lifestyle. Although, there is now also growing evidence of increasing rates of disease in rapidly developing countries, showing a correlation with rising economic growth and changes in diet and lifestyle [4]. Numerous studies indicate that these types of allergic responses often occur in a progressive manner termed the “atopic march,” initially presenting early in infants as a skin allergy or eczema that is linked to an underlying food allergy [5]. Subsequently, many children go on to become sensitized to indoor allergens, such as dust or pet dander and to develop allergic rhinitis and then asthma later in childhood or in their early teenage years [5]. Sensitization to outdoor aeroallergens such as grass and tree pollens typically occurs during the later phases of the atopic march, at a time where sensitization to food allergens may be seen to decrease [6]. The presence of atopy early in life has been shown to significantly increase the risk for development of additional sensitizations, resulting in a progressive form of atopic disease that advances in an additive fashion [7]. Children initially presenting with atopic dermatitis, the most commonly diagnosed form of atopy within the first 6 months following birth show increased risk for the development of asthma and allergic rhinitis, with the incidence of subsequent disease being associated to the severity of the initially diagnosed atopic dermatitis [5]. These findings imply that certain individuals are predisposed to the development of atopic disease, and early age of onset may be indicative of a susceptible phenotype predictive of increased risk for multiple sensitizations [7]. Many risk factors are associated with the onset of atopic disease, including parental history of atopy [8], breast milk vs. formula feeding [9,10,11,12], diet [13], air pollution [14], use of antibiotics [15,16,17], and mode of delivery [18,19,20], having been well characterized through epidemiological studies. Whereas data describing the mechanisms linking these environmental factors with the aberrant activation of the adaptive immune system that is responsible for the onset of disease have lagged behind.
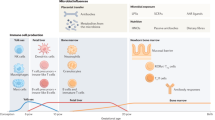
The adaptive immune system plays a pivotal role in the development of defense against potential infectious pathogens [21] and as the primary function of the adaptive immune system is to protect against invading pathogens, immune responses generally have an inflammatory effect with potential immunopathological consequences that need to be tightly controlled. To identify potential pathogens, the adaptive immune system requires the ability to distinguish between self and non-self-antigens, whilst simultaneously discerning harmless environmental antigens which can be safely ignored [22]. Occasionally, a failure in the system of checks and balances that is required to maintain immune tolerance occurs, resulting in either autoimmune disease elicited against self-antigens or development of allergic disease against otherwise harmless environmental antigens [23], with studies demonstrating the influence of genetic, developmental, and environmental factors, all contributing to the breakdown of immune tolerance that causes disease [24,25,26,27]. Although, significant variations occur, the majority of allergic responses are manifested in early childhood when the immune system is still developing [28,29,30]. Here, the role of T cells in the initiation of an allergic response has been widely studied, with helper T cells, particularly cells of the Th2 lineage being characterized as the major mediator involved in eliciting allergic responses. Subsequently, it has been determined that a balance between inflammatory Th2 and suppressive Treg cells exists that controls the threshold for allergen sensitization [31] (Fig. 1).
Influences of environmental and microbial interactions on adaptive immune responses and allergic disease. A wide array of factors including, genetic, environmental, and dietary inputs can all potentially modulate the gut immune-microbiome axis and influence the occurrence of allergy. The microbiome in turn modulates the cohort of regulatory cells induced during development and allows for the establishment of a tolerogenic environment, which mediates the suppression of T cells that arise from inflammatory lineages. However, a dysbiosis of the microbiome leads to impairment of this tolerogenic environment leading to development of allergic diseases along with greater expansion in cells of the Th2 inflammatory lineage
The recent development of “omic” sequencing techniques allowing for the rapid and affordable sequencing of the microbiota has led to studies that have revealed the relevance of the microbiome as a key environmental factor [32, 33]. Allowing us to begin to elucidate how exposure to the natural microbial environment influences the development of the immune system, especially at sites integral for barrier immunity, with new data indicating that host-microbiome interactions during early development play a significant determining role in shaping the immune responses of the host [34, 35].
Microbial Exposures and the Development of Allergic Disease
Atopic Dermatitis
Atopic dermatitis, otherwise known as atopic eczema, is an inflammatory skin disorder which mostly develops in childhood and is characterized by itchy eczematous lesions [36]. Atopic dermatitis affects nearly 15–20% of children and 1–3% of adults worldwide [37, 38]. Multiple risk factors contribute to the development of atopic dermatitis, which has a close association with food allergy, particularly in early childhood. The earlier the onset of atopic dermatitis, the higher the risk of food allergy, particularly in relation to peanut, cow’s milk, and hen’s egg allergens [36]. Patients with atopic dermatitis exhibit an increase in the skin pH and lipid deficiency during disease flares, along with the degradation of the skin barrier function [39].
Recently, the skin microbiome has been identified as a critical factor in the development of atopic dermatitis (Table 1) [40,41,42,43]. Microbial diversity has been described to have an inverse correlation with atopic dermatitis score (SCORAD), with decreased microbial diversity at sites of disease progression [44]. Specifically, an increase in the relative abundance, particularly of Firmicutes (Gemella spp., Staphylococcus aureus [44] and Staphylococcus epidermis and a decrease in the abundance of the phylum Actinobacteria (Dermacoccus spp.) [45] and Proteobacteria [34] and the genera Streptococcus, Corynebacterium, and Cutibacterium has been associated with atopic dermatitis [34]. Byrd et al. showed that a greater predominance of Staphylococcus aureus occur in patients with more severe disease and Staphylococcus epidermidis is predominant in patients with less severe disease [46]. Staphylococcus aureus strains isolated from patients with atopic dermatitis were enriched with the CC1 strains, whereas the healthy control population was enriched with the CC30 strains [47]. Additionally, topical colonization of mice using strains isolated from patients with atopic dermatitis or controls showed that Staphylococcus aureus isolates from patients with more severe disease flares are capable of inducing epidermal thickening and expansion of cutaneous Th2 and Th17 cells, indicating that functional differences in these distinct staphylococcal strains can contribute to the complexity of atopic dermatitis [46].
In addition to the role of the skin microbiome, the composition of the gut microbiome was similarly found to be altered in patients with atopic dermatitis who exhibited a low gut microbial diversity which was also associated with disease [48,49,50,51,52,53,54]. The composition of the gut microbiome in 1-month-old infants from the KOALA birth cohort study showed that colonization with Clostridium difficile led to an increased risk of development of atopic dermatitis and other allergic diseases [33]. Furthermore, atopic dermatitis has been associated with an increased abundance of Firmicutes, specifically the Clostridium difficile, Coprobacillus spp., Enterococcus spp., and Peptoniphilus spp. and a decreased abundance of Proteobacteria and Bacteriodetes in the intestine [54, 55], potentially due to a lack of exposure to the LPS contained in the cell walls of Proteobacteria, which exerts a protective action through boosting IL-12 production by monocytes and dendritic cells to induce responses [56], which may otherwise be impaired in pediatric atopic dermatitis patients [55]. Bacterial metabolites synthesized by the gut microbiome also play a pivotal role in providing protection against the development of atopic dermatitis [57]. As the intestinal barrier function can be impaired in those patients [58], with impairment often being associated with an enrichment of Fecalibacterium, in particular, Fecalibacterum prausnitzii, a non-short chain fatty acid (SFCA)-producing bacteria which is especially common in pediatric atopic dermatitis [59] and can lead to an increased inflammation of the gut epithelium in these patients [60]. Alternately, SCFA-producing bacteria can upregulate the expression of tight junction to improve the intestinal barrier function [55]. Moreover, SCFAs like butyrate can regulate the activation and proliferation of colonic Treg cells, which has been shown to be protective in mouse models of disease [57, 61].
Atopic Food Allergies
Food allergy is a condition that has shown a significant increase in prevalence over the last 20 years [62], now affecting nearly 8% of children and 5% of adults worldwide [63]. The eight most common food allergens in young children are cow’s milk (2.5%), egg (1.3%), peanut (0.8%), wheat and soy (nearly 0.4% each), tree nuts (0.2%), and fish and shellfish (0.1% each) [64]. Food allergies can be broadly classified as resulting from immune pathways that activate effector cells through food-allergen specific IgE or non-IgE-mediated mechanisms, [64, 65]. Oral tolerance is induced under homeostatic conditions and leads to the suppression of immune responses to food-derived foreign antigens encountered in the gastrointestinal tract [66]. The loss of oral tolerance initiates a cascade of immune responses against otherwise innocuous food antigens resulting in food allergy [67]. As at other sites of barrier immunity, Treg cells play a key role in maintenance of tolerance by regulating immune responses to allergens through several mechanisms, including the production of the inhibitory cytokines IL-10 and TGF-β [68], by inhibiting the proliferation of effector T cells, depriving the cells of IL-2 [69] and through the production of granzymes A and B that can cause cytolysis of effector T cells [68]. Several recent findings suggest that the SCFA, butyrate, contributes to the development of oral immune tolerance due to its strong anti-inflammatory effects [70, 71]. Emerging 16S rRNA sequencing–based studies on food allergy and sensitization indicate that gut dysbiosis may precede the development of food allergy (Table 1) [72,73,74,75,76]. The Canadian Healthy Infant Longitudinal Development (CHILD) study indicated that a reduced gut microbial diversity at 3–6 months was associated with an increased tendency for food sensitization at 12 months and showed an increased abundance of Enterobacteriaceae and a decreased abundance of Bacteroidaceae and Ruminococcaceae [77]. A US pediatric cohort study determined that a lower abundance of Citrobacter, Oscillospora, Lactococcus, and Dorea in stool samples collected at 3–6 months of age was associated with food allergy by age three and a relatively lower abundance of Haemophilus, Dailister, Dorea, and Clostridium in stool samples of the same age group exhibited food sensitization by age 3 to at least one of the eight major food allergens [78]. Whereas clostridia exhibits a protective effect against sensitization to food allergens through regulation of the innate lymphoid cell function and intestinal cell permeability [55]. Although the gut microbiome changes with time, the most rapid changes occur early in life and are mainly influenced by whether the infants are vaginally delivered or via Cesarean section (C-section) and breast- or formula-fed [79]. In addition, antibodies, such as IgA at the mucosal surface of the intestine, can diffuse across the gut epithelium into the lumen to bind and prevent an inappropriate crossing of intestinal microbiota into the bloodstream [80]. With IgA levels being essential for the maintenance of intestinal homeostasis and the regulation of gut microbiota composition [81], especially during the post-natal period where IgA is transferred to infants through maternal breast milk and plays a vital role in immune and microbial homeostasis. Intriguingly, a recent study by Abdel-Gadir et al. [82] reported a decrease in binding of fecal bacteria to IgA and an increased binding to IgE in infants with food allergy revealing a previously undescribed allergic response to commensals in the intestine of food allergic patients.
Asthma and Allergic Rhinitis
Asthma is a common allergic inflammatory disease affecting more than 300 million people worldwide [83]. Broadly, asthma can be defined to be of either an atopic or non-atopic phenotype. In atopic asthma, the incidence of asthma symptoms occurs later in childhood or in early teenage years and may be resultant of an underlying genetic predisposition to allergen-sensitivity leading to development of hyper-responsiveness with symptoms mostly persisting into adulthood [84]. Whereas, non-atopic asthma largely develops within the first 2 to 3 years of age and develops as a neutrophil associated, recurring obstruction of the airways that typically resolves by around 13 years of age [84]. Several studies have reported the positive influence of microbial exposure on protection from the development of asthma, with children who are exposed to a highly diverse microbial environment often exhibiting lower rates of asthma and allergic rhinitis (Table 1) [24, 85,86,87,88,89,90]. For instance, in rural areas, children raised on farms are more likely to be exposed to livestock, as well as an increased likelihood of having consumed unpasteurized milk from farm animals during their early childhood [25, 91]. Such exposures to a microbial environment at a very young age are associated with a relatively lower risk of developing allergic diseases [92]. This is typically referred to as the “farm-effect” on allergic diseases and has been associated with both atopic and non-atopic phenotypes of asthma [92, 93]. Schuijs et al. reported that the ubiquitin-modifying enzyme A20 in the lung epithelium renders the protective effect in children living in farm environment, showing that the loss of A20 enzyme eliminated the protective effect; in addition, a single nucleotide polymorphism in the gene encoding for A20 enzyme was associated with allergy and asthma risk in children raised in a farm environment [94]. It was further shown that farm dust and bacterial LPS modify the communication between epithelial cells and dendritic cells, achieved through the induction of A20 expression [94], providing a possible explanation for the incidence of higher rates of asthma in children from urban areas, as compared to those from rural areas. Allergic rhinitis, also known as hay fever, is an atopic disease characterized by nasal congestion, sneezing, and rhinorrhea [95] and is predominantly caused by allergens such as pollen, dust mites, and animal dander; based on the causative allergen, allergic rhinitis can present as either seasonal or perennial in nature [96]. A recent analysis of the microbial composition in the mucosal airways of children with asthma or allergic rhinitis identified a decrease in certain groups of microbes and linked this microbial dysbiosis with the increased sensitization to allergic disease [97, 98]. Among those microbes found to constitute a healthy lung microbial composition were Bacteroidetes, Actinobacteria, and Firmicutes [83], whereas the phylum Proteobacteria was found to be abundant in asthmatics and was associated with lower levels of asthma control and increased numbers of asthma exacerbations [99, 100]. Other common microbial populations found in the mucosal airways of asthmatic patients include Prevotella, Selenomonas, Butyrivibrio, [97], and Neisseria [97, 101]. Some of these species, which includes Prevotella and Neisseria species, are also found associated with patients with allergic rhinitis [97]. Interestingly, the airway microbiome composition in patients with eosinophilic and neutrophilic asthma was found to be distinct, with the neutrophilic patients exhibiting a reduced diversity and richness in Proteobacteria and in particular, Haemophilus and Moraxella species [102, 103]. Haemophilus parainfluenzae is capable of activating the toll-like receptor (TLR) 4, which in turn leads to the transcription of pro-inflammatory cytokine IL-8 and inhibition of corticosteroid responses [104]. Apart from the airway microbiome, dysbiosis of the gut microbiome and lower gut microbial diversity early in life are also associated with a subsequent increased risk of developing asthma [105]. Data from the CHILD cohort study which examined children who were asthmatic at 4 years of age showed that the gut microbial composition of these children at 3 months of age exhibited a significant decrease in the abundance of the genus Lachnospira and an increased abundance of the species Clostridium neonatalae [106]. A further study which analyzed the gut microbial composition of infants at risk for asthma, during the first 100 days of their life, showed a decreased abundance of Lachnospira, Veillonella, and Faecalibacterium from the phylum Firmicutes and Rothia from the phylum Actinobacteria [85]. Yet another study showed that children with asthma had a significantly lower abundance of Faecalibacterium and Roseburia, belonging to the phylum Firmicutes, whereas Enterococcus and Clostridium from the same phylum were enhanced in these children as compared to healthy controls [107].
Early Life Exposures to Microbes and Immune System Development
Pre- and Post-Natal Colonization of Barrier Organs by the Microbiota
Until recently, it had been assumed that the prenatal environment was a sterile location, free from microbes. However, several studies published over the last decade have begun to question the “sterile womb” theory and whether prenatal colonization of the developing fetus does in fact occur [108,109,110,111,112]. The concept of a prenatal microbiome remains highly controversial and has been extensively reviewed [113, 114] and debated previously [115, 116]. New findings published in Mishra et al. [117] may present the best evidence for microbial exposure and colonization of fetal organs by the microbiome. The authors showed that fetal organs contained a diverse array of bacterial species and that bacteria isolated from these organs can be grown under in vitro culture conditions. Bacterial structures were additionally visualized by electron microscopy in the 14-week-old fetal gut, with coincident staining for the presence of 16S rRNA by RNA in situ hybridization. Analysis of the T cell compartment revealed the presence of fetal memory T cells that were able to be expanded in the presence of the fetal-isolated bacterial strains. Whether these findings represent the identification of a definitive fetal microbial niche, indicative of a true host-microbe relationship or are merely evidence of persistent or transient colonization, still remains to be determined. However, evidence for the microbial priming of an adaptive immune response during the period of fetal growth has significant implications for the development of the immune system and may play a considerable role in the development of atopic disease susceptibility that has yet to be determined.
The exposure to the microbial environment early in post-natal life plays a significant role in the development of the immune system (see Table 2) [24]. The first few days after birth when neonates get their first major microbial inoculation represents a critical window in their immune system development [9, 118]. During this window, several factors can alter or influence the initial microbial colonization [24]. Among the potential factors, mode of delivery provides an initial strong primary determinant for post-natal colonization of microbial communities and associated barrier functions, playing a major role in the development of the subsequently established microbiome. During vaginal delivery, neonates acquire the major microbial communities from the mother, mainly characterized by an increased abundance of Bacteroides and Parabacteriodes [119]. In contrast, babies born via C-section receive their first microbial inoculum from other sources, such as skin, saliva, or breast milk [119]. Interestingly, emerging evidence indicates that microbial communities acquired via vertical transmission are capable of adapting quickly to the new environment, and priming the associated immune functions, particularly LPS biosynthesis pathways and two-component systems pathways that are significantly under-presented in neonates born via C-section [120]. LPS, is a membrane component of Gram-negative bacteria and is capable of priming the neonatal immune system by stimulating secretion of pro-inflammatory cytokines at the interface of the earliest gut microbiome, which may result in persistent effects on neonatal physiology, including protective effects towards developing allergies later in life [86, 121]. On the other hand, an early perturbation of the host-commensal priming in neonates born via C-section can lead to defects in the proper education of the immune system [122] and higher propensities to develop chronic diseases later in life [123], with significant increases in the incidence of antibiotic resistant, hospital associated microbes being detected in several studies [124,125,126].
Apart from the mode of delivery, other environmental factors such as feeding with breast milk, staying in a joint family, or farm environment in the first few years of life can increase exposure to vast microbial diversity (Fig. 1), which may result in adequate immune responses against diverse microbial antigens; however, elimination of such microbial exposures either by feeding with formula milk, staying in a nuclear family, or exposures to antibiotics at a young age can promote inflammatory immune responses including those associated with asthma and other allergic diseases [24].
Pre- and Post-Natal Development of the Adaptive Immune System
During pregnancy, the maternal immune system adopts a complex immunologic mechanism to enable the co-existence and maintenance of an equilibrium between both the maternal and developing fetal immune systems [13, 127]. In order to prevent fetal and placental immune rejection, whilst allowing for the unmatched tissue growth of the fetus as it prepares for adaptation to the external environment and the ensuing microbial colonization that occurs at birth [128]. The thymus becomes a functional organ of T cell development between the 7th and 16th week of gestation [129]. During the 8th week of gestation, early lymphoid progenitors originate from the liver and subsequently migrate into the thymus where they develop into naïve T cells [130]. Circulating T cells are observed around the 10th to 11th week of gestation following the development of a functional thymus [131]. Impaired growth of the fetal thymus has been shown to be related to several complications associated with pregnancy including preeclampsia, a condition which presents with reduced peripheral Treg cells in both the mother and newborn [132] and which in turn is associated with increased risk of allergic diseases development during childhood [133]. The fetal immune system is generally characterized as tolerogenic [134, 135], a feature essential for fetal survival [136]. During the gestational period, a substantial number of maternal cells cross the placenta to reside in the fetal lymph node which provokes the development of CD4+CD25hiFOXP3+ Treg cells. Various cytokines, hormones, and bacterial products including SCFAs and lipopolysaccharides are also involved in transplacental immune regulation [132, 137]. Fetal Treg cells suppress the proliferation and cytokine secretion of other potentially self-reactive T cells [134]. Human cord blood and infant blood are both characterized by a predominance of Th2 and Treg cells, as compared to Th1 or Th17 cells, which are more restricted in early life [134]. In fact, the Th2 and Treg phenotype bias in fetal tissues develops as early as the second trimester of pregnancy as identified from fetal spleen and lymph nodes [135]. A recent study described the differential expression of arginase-2 between fetal and adult splenic dendritic cells resulting in the fetal dendritic cells to favor the induction of Treg and Th2 cells over Th1 cells [138]. Human cord blood studies have also shown that neonates have negligible amounts or complete absence of Th17 cells [139] that play significant role in developing immunity against fungal and bacterial infections at epithelial barriers [140]. Tregs and Th17 cells have reciprocal development pathways in the absence of the pro-inflammatory cytokines IL-6, IL-1β, and IL-23 wherein Foxp3 dominates RORγt function and prevents Th17 development. Thus, neonatal immunity is typically considered to exhibit an anti-inflammatory profile as the Th2 and Treg phenotype bias in early life impairs Th1 and Th17 immune responses [141] but may instead lead to a bias towards the development of an atopic phenotype.
Mechanisms of Microbial Effects on Adaptive Immunity
Immuno-Microbiome Interactions Alter T Cell Differentiation in Allergic Diseases
The human body is constantly exposed to an external microbial environment comprised of a wide variety microbial species able to colonize the barrier organs of the human body forming symbiotic, commensal, or pathogenic relationships with the host [142, 143]. During development the immune system co-evolves in the presence of these microbes and plays an important role in maintaining the homeostatic balance between tolerance and allergy, through regulation of host-microbiome interactions. Recently, the influence of the gut microbiota in the development of allergic disease has become widely studied, revealing that during early developmental stages, the immune system becomes tolerized to commensal bacteria, through the induction of a corresponding complement of IgA antibodies and regulatory T cells, enabling the commensal bacteria present to be maintained in the gut over time [35]. Interestingly, it has been shown that compositionally and functionally distinct gut microbiota exist at different stages of neonatal development and exert differential influences on the immune system [32]. During development, gut microbial dysbiosis may occur which can alter the homeostatic balance of the immune system leading to a cascade of events that results in an allergic outcome [78]. Animal-based studies have identified the presence of at least two distinct types of Tregs in the gastrointestinal tract — thymically-derived Treg (tTreg) and peripherally induced Treg (pTreg), the latter being predominantly enriched in the intestines and largely responsible for maintaining tolerance to food and microbiota-derived antigens at mucosal surfaces [144], with the composition of the gut microbiome influencing the differentiation of pTregs in an antigen-specific manner [145]. Germ-free animal studies indicate that pTregs fail to differentiate in the gastrointestinal tract of mice lacking a diverse microbiota, whereas a normal tTreg compartment was maintained in these animals [144]. Furthermore, it has been reported that dietary antigens can induce the differentiation of pTregs, with this population being distinct from the microbiome-induced pTregs, in that they co-express both Foxp3 and GATA3, have a limited life-span, and can repress the strong immunity to ingested protein antigens [146]. Although most of these studies have been conducted in animal models, recent clinical studies on human subjects identified that Tregs are not functionally impaired in individuals with allergies, but there is a striking increase in the proportion of reactive Th2 cells in these individuals [147], indicating that antigen escape from Treg control was the dominant factor associated with the loss of tolerance observed in allergic individuals.
Th2/Treg Axis and the Microbiome
Under homeostatic conditions, the adaptive immune system normally develops a system of tolerance to harmless environmental antigens largely mediated by members of the T cell lineage, particularly Treg cells [147]. Tregs play a dual role in the intestine, by maintaining immune tolerance to dietary antigens [148] and limiting the potentially damaging immune responses that can be generated against environmental pathogens [149]. However, when there is a failure in generation of tolerance by Treg cells against common food and aero-antigens, it can result in the differentiation of allergen-specific cells of the Th2 lineage and the associated production of atopy-inducing IgE [150]. Along with a cascade of downstream events triggered by production of cytokines including IL-4, IL-5, IL-9, and IL-13, ultimately leading to the recruitment and activation of a raft of innate immune cells including basophils, eosinophils, ILC2’s, and M2 macrophages, which together cause the pathology associated with allergic disease [151]. A subset of human memory Th2 cells, allergen specific Th2 cells have been recently reported to be confined only to atopic individuals, referred to as Th2A cells. These Th2A cells are terminally differentiated and co-express CRTh2, CD49d, and CD161 and are thought to be functionally distinct from conventional Th2 cells [152], as they may be more readily activated by perturbations in mucosal barrier function and activation by the “alarmin” cytokines TSLP, IL-25, and IL-33 [153]. Treg cells, on the other hand, produce the anti-inflammatory cytokines, TGFβ, and IL-10, which among other regulatory mechanisms are key in protecting the host from excessive inflammatory immune responses [154]. Hence, a balance in the Th2/Treg axis is essential to protect against the development of allergic disease [155]. Studies in animal and human systems have begun to elucidate several potential mechanisms that tie the microbiome and microbial diversity to alterations in the regulation of Th2 and Treg differentiation leading to a cellular and molecular rationale for those observations made by the hygiene hypothesis. Intriguingly, studies of germ-free mice lacking a microbiome found that a complete microbial dysbiosis led to the establishment of a default Th2 biased immune environment [156, 157], reminiscent of that observed during neonatal and early post-natal life [134, 135, 138]. Indicating that the introduction of commensal bacteria at an early stage may be critical for ensuring normal cellular maturation and recruitment in order to control allergic inflammation. Studies into the relative roles of tTreg and pTreg cells revealed that animals deficient for pTreg had altered ratios of Firmicutes to Bacteroidetes, which was associated with the spontaneous induction of Th2-associated mucosal inflammation and lung pathology [158]. Revealing that pTreg are essential for regulation of the homoestatically controlled microbial community in the gut, through exerting control over Th2 mucosal inflammation and regulating the induction of Th17 differentiation which enables B cell production of IgA and the establishment of a “mucosal firewall” [159]. A key component of this microbial regulation of type 2 inflammation was shown to be induced through the induction of intestinal RORγt + pTregs, which modulate the differentiation of Th2 cells in a CTLA-4-dependent manner by regulating the co-activator functions of stimulatory DC [160], thereby balancing immune responses at the mucosal surface through the induction of Th17 and Treg cells [145].
Effects of Microbial Products on T Cell Immune Responses
Multiple studies have now shown that specific products of metabolism synthesized by the component species of the microbiome are able to exert effects on the differentiation and function of CD4 + T cells [161], providing a mechanistic rationale that directly links microbial dysbiosis with the alterations in immune function that lead to allergic disease (Table 3). Some of the earliest studies linking microbial metabolites with alterations in immune function focused on the effects of antibiotics on disrupting microbial homeostasis, as the use of antibiotics at an early age is a known risk factor associated with increased risk for atopy [161]. Murine models have revealed that along with the induction of a microbial dysbiosis, antibiotic treatment also caused a concomitant decrease in protective gut Treg cells and the induction of inflammation. Intriguingly, this decrease in Treg cells and the associated inflammatory response could be inhibited by treatment with SCFA [162] (Fig. 2). Under homeostatic conditions, SCFA are produced by the microbial conversion of dietary fiber by anaerobic fermentation [163], especially by those microbial taxa associated with protection, including Clostridia and Firmicutes.
Microbial Product Signaling Influences T Cell Immune Responses in Homeostasis and Dysbiosis. A A diverse microbiome promotes homeostatic maintenance and the induction of immune tolerance. Crosstalk between the microbiome and the immune system is mediated by microbial products that promote the differentiation of pTreg and the upregulation of RORγt in response to stimulation with food derived antigens. B Microbial dysbiosis results in the production of microbial products that promote inflammatory responses and modulate Th2 differentiation in response to the presentation of Ag’s and stimulate ILC2 recruitment, resulting in type 2 inflammation in affected tissue following the recruitment of innate cell mediators of disease including mast cells and eosinophils
Generation of protective intestinal pTreg can be driven by SCFA including butyrate [145] and propionate [160] in conjunction with bacterial antigens [164]. Generation of the type 2 response suppressing RORγt + Tregs was found to be largely dependent on the presence of SCFA butyrate [61, 165], providing an intriguing molecular post-biotic link between the microbiome and regulation of inflammation. Butyrate has also been shown to play a distinct role in the regulation of type 2 innate lymphoid cells (ILC2s), which are associated with the induction and exacerbation of asthma. Here, butyrate was found to alter HDAC2 activity inhibiting GATA3 expression and ILC2 proliferation [166]. It should be noted that whilst SCFAs contribute to mucosal immune homeostasis, excessive or suboptimal levels of SCFAs have been reported to be associated with inflammation and cancer [167]. The microbiome has also been observed to modify a range of host-derived molecules into steroidal compounds with de novo biological activities and immune functionality [160].
Bile acids which usually aid in the emulsification of dietary fats can also undergo bacterial transformation in the colon into the secondary bile acid 3β-hydroxydeoxycholic acid, which in turn can modulate DC function and facilitate the differentiation of, and increase in the levels of RORγt + pTregs present in the intestinal mucosa [168] that are key for regulating the onset of spontaneous type 2 inflammation [169]. The activation of DC by microbial polysaccharides acting through Toll like receptor-2 (TLR2) signaling has also been demonstrated to play an anti-inflammatory role in the intestine via the induction of Treg and an increase in local IL-10 production [170, 171]. The importance of the B vitamins folate (B9) [172] and niacin (B3) [173] in the maintenance and regulation of function in the intestinal Treg compartment has previously been described, along with studies linking folate with both protective effects against asthma [174] and increased rates of food allergy [175]. Hence, future studies are needed to confirm the direct mechanistic action of these metabolites in the context of allergic disease. Colonization of the gut with L. reuteri was previously shown to induce protective Treg cells in an allergic airway mouse model [176]. More recently, studies have indicated that a tryptophan metabolite Indole-3-lactic acid (IDO) produced by both L.reuteri [177] and B. infantis were able to silence both Th2 and Th17 cells through upregulation of galectin-1, demonstrated in human studies [178].
In a longitudinal study focused on multisensitised atopic children, it was determined that alterations in the gut microbiome and in metabolic activity were evident as early as 3 months of age, during which a distinct fecal microbiome and metabolome were present in those children who went on to develop atopy at 3 years of age. Fecal metabolite extracts isolated from these subjects induced an increase in the relative proportion of IL-4 expressing Th2 cells, whilst also exhibiting Treg suppressive capabilities [168]. Subsequently, one of the compounds identified in this study, 12,13-diHOME a linoleic acid metabolite, was shown to exacerbate lung inflammation in mice and elevated levels of the compound were detectable in neonatal children at 1 month of age, who later went on to develop atopy by age two [169], providing further evidence of the importance of the gut microbiota and the microbial products that it produces in conditioning of the immune system at an early time in life.
Microbial Dysbiosis and the Development of Allergic Diseases
The Hygiene Hypothesis
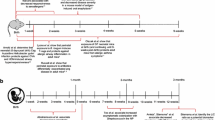
The “hygiene hypothesis” concept dates back to a longitudinal study published by Strachan in 1989 [179] and was initially put forward as an explanation for the emergence of hay fever as a “post-industrial revolution epidemic.” The data published in Strachan’s study established a correlation between hay fever and house size and noted that as the number of older children in the house rose, the incidence of hay fever in younger siblings decreased, leading to the hypothesis that allergic diseases may be associated with a lack of early childhood exposure to infectious disease, spread by unhygienic interactions with older children. Hence, a general decrease in family size and increase in personal cleanliness, along with a concomitant decrease in exposure to Th1 skewing infections during early childhood caused the increased rates of atopic disease [180]. This initial correlation between rates of early childhood infection with the incidence of atopy was subsequently assessed more directly in multiple studies. An analysis of herpes simplex virus infection rates during the first 3 years of life indicated that infection was protective against asthma [181] and a Brazilian study indicated that higher infectious burdens during early life, as measured by plasma Ig levels for exposure to multiple pathogens including herpes simplex virus, Epstein-Barr virus, Toxoplasma gondii and Helicobacter pylori, correlated with lower levels of atopy [182]. Additional studies of tuberculosis infection [180], varicella infection [183], and BCG vaccination [183] also found an association with lower rates of atopic disease. These findings indicate that early life exposures to bacterial and viral infections and the production of a strong Th1 response were important in promoting a protective environmental milieu that skews the immune system away from the development of an atopic disease inducing Th2-biased system (Fig. 3).
Cellular Basis for Hygiene Hypothesis and Old Friends/Biodiversity models. A The original hygiene hypothesis focused on an absence of a sufficient viral/bacterial pathogenic burden to educate the immune system in childhood, allowing for the induction of an aberrant Th2 response. Mechanistically this was seen due an imbalance in the reciprocal regulatory relationship that exists between Th1 and Th2 responses mediated by IL-4 and IFN-ɣ. B Old friends/biodiversity hypotheses’ additionally factors in the presence of regulatory mechanisms that are essential for control of both autoimmunity and allergic responses and are associated with the presence of a diverse microbiome. Here, the lack of sufficient regulatory responses accounts for the parallel rise in both autoimmune disease (Th1) and allergy (Th2) seen in recent history
However, several arguments against this Th1-Th2 cytokine shift paradigm have emerged. Large-scale and longitudinal cohort studies from the UK [184], Denmark [185], and Finland [186] concluded that after adjustment for clinically apparent infectious diseases, a protective effect of number of siblings, day care, pet ownership, and farm residence was instead responsible for the decreased odds ratios observed (Table 4). Additionally, as atopic disease rates have increased, a concomitant rise in cases of autoimmune diseases has also been observed in children. Including diabetes mellitus (T1D), Crohn’s disease, and multiple sclerosis [187]. As these diseases are largely dominated by Th1 and/or Th17 responses [188], it seems unlikely that the Th1-Th2 cytokine shift paradigm is robust enough to explain the increased development of both sets of disease. Especially in light of findings that indicate there may in fact be an association between the incidence of allergic and autoimmune diseases [189,190,191]. Furthermore, a large-scale retrospective cohort study (1990–2018) conducted in the UK concluded that the long-term risks of autoimmune disorders are significantly higher in patients with allergic diseases [192]. A recent meta-analysis of the commonalities in 290 genetic loci previously associated with 16 autoimmune diseases, found a significant enrichment of multiple loci also associated with allergy, suggesting that a further investigation of shared mechanisms may help in understanding the complex relationship between these disease syndromes [193]. Contrary to what might have been predicted by the Th1-Th2 cytokine shift paradigm, infections with helminths result in a Th2-polarized immune response including production of IL-4, IL-5, IL-13, eosinophilia, and high serum titers of IgE, all hallmarks of allergic disease. Whilst at the same time, helminthic infections have largely been associated with inducing protective effects on the development of atopic disease as well as naturally occurring infection with Trichuris trichiura, Enterobius vermicularis, and Schistosoma mansoni, which have all been shown to exhibit a protective effect [194], especially when exposure was found to occur during in utero development [195] or early in life [196, 197]. However, the protective effects of helminths do not appear to be universal, for example, infection with Ascaris lumbricoides or S. mansoni has also been associated with higher asthma rates in certain populations [198,199,200].
Although, the “hygiene hypothesis” has been widely accepted by both the scientific community and the general public, it is not without its limitations. Care needs to be taken in terms of interpreting the message when associating hygiene with the pathogenesis of atopic disease [201]. The hygiene hypothesis has however been pivotal in framing the idea that the immune system is still relatively naïve at birth and whilst the adaptive immune system has gone through an internal developmental process to largely limit the number of cells present capable of mounting a response against self, it still needs to be “fed” with information about how to interpret antigens present in the local environmental. Recent studies indicate that this process may largely occur within the first few months following birth through constant contact with microbes from the external environment, as well as via transfer from other humans, especially from close maternal contact. When such microbial exposure is inadequate, the mechanisms regulating the immune system can fail, resulting in autoimmune and allergic diseases [202].
Current Perspectives
The “old friends” hypothesis, put forward by Rook in 2003 [203], and the “biodiversity hypothesis” of allergy proposed by Haahtela [204] have subsequently emerged, both of which postulate a similar theme that the emergence of allergic reactions is an outcome of a lack of symbiotic relationships with parasites, viruses, and bacteria which have been beneficial for evolution in the past [205]. These hypotheses are also sometimes referred to as “Western lifestyle hypotheses.” The western lifestyle is being generally characterized by minimal or no physical activity among children with most of the time being spent indoors leading to obesity among children and also exposure to increased allergenic burdens, especially those found indoors, including house dust mites (HDMs) [205], thereby leading to a massive shift in the human disease spectrum from infectious diseases to allergies. In addition, excessive use of antibiotics, with an average of approximately 2.5 antibiotic doses per 100 people/day in Western countries [206], and increased use of sanitation technologies have resulted in elimination of certain eukaryotic symbionts, including helminths and protists from the human gut ecosystems [207]. Taken together, these environmental factors associated with westernized lifestyle trigger dysbiosis by affecting intestinal epithelial cell metabolism, sequestering nutrient sources [208], and creating a favorable environment for facultative anaerobes-such as pathogenic Escherichia coli and Salmonella [209, 210] at the expense of symbiotic flora such as Bacteroides, Prevotella, Desulfovibrio, and Lactobacillus [211, 212]. The microbial dysbiosis induced by a westernized diet or lifestyle may also result in a leaky mucosa and reduced intestinal production of short chain fatty acids (SCFAs) [213]. A prolonged microbial dysbiosis may lead to leakage of pathogen-associated molecular patterns (PAMPs), including LPS, in the blood, and trigger low-grade inflammation or allergy [213, 214], ultimately resulting in a change in the lung, gut, and skin microbiomes causing microbial dysbiosis which leads to a sharp decrease in infectious diseases and a higher prevalence of allergies (Fig. 3).
“Unhygienic Therapies” for Atopic Diseases
As the “hygiene hypothesis” postulates that a lack of microbial interactions in early life leads to an increased risk of atopic disease, the reverse correlate of this implies that the introduction of microbes or use of “unhygienic therapies” may be beneficial in restoring the missing constituents of microbial communities for either treatment or prevention of disease (Tables 5, 6, and 7). The treatment of immunocompromised patients suffering from recurrent Clostridium difficile infections has demonstrated the amazing utility of fecal microbiota transplants, where fecal transplant has revolutionized the management of disease leading to a cure rate of 90% after treatment [215]. Although there have been limited studies to date, looking at the use of fecal transplant for treatment of allergic disease in humans [216], a recent study has demonstrated that the transfer of fecal material from healthy infants into a germ-free mouse model was protective against an anaphylactic response to cow milk allergens, whereas colonization of the murine gut with the microbiome from cow’s milk allergic infants was unable to confer protection [217]. Additionally, the transfer of the skin microbiome has become a recent area of interest with the topical microbiome transplantation of R. mucosa demonstrating efficacy for treatment of both pediatric and adult atopic dermatitis [218] and more recently, the use of bacteriotherapy was shown to decrease the incidence of S. aureus in AD patients [219]. Whilst these studies do provide hope for use of microbial treatment for allergic disease, a large number of studies have examined the possibility of using probiotic supplementation to either prevent or treat disease (Table 1). The majority of probiotic studies into food allergy and atopic dermatitis have investigated administration of either a single or the combination of a limited number of bacterial species and have been shown to have limited utility in either preventing or treating allergic disease [220,221,222,223,224,225,226,227,228]. Probiotic intervention strategies have also been widely trialed as a potential prophylactic therapy for asthma both during pregnancy [229] or during infancy [230,231,232]. However, the meta-analysis of these studies has not revealed any substantial protective benefits which could be derived from the current probiotic therapies trialed.
Exposure to infections with helminthic nematodes is also largely absent in industrialized societies, raising the prospect for an additional therapeutic avenue to be explored. Parasitic worms or compounds from their excretory/secretory milieu could potentially play a role in mediating tolerance induction either by affecting the composition of organ specific microbiomes or through direct action on the immune system [233]. Infection during early life (0–5 years) with the human parasite T. trichiuria has been shown to significantly reduce the incidence of allergy later in life [234], potentially through an early life imprinting of the immune system towards a tolerogenic phenotype, much in the same way that early atopic symptoms can be indicative of initiation of the atopic march. Anecdotal accounts in the news media of individuals self-curing themselves through helminth infections have created much interest in helminthic therapy [233, 235]. However, randomized controlled trials into the efficacy of helminthic treatment for asthma [236], hay fever [237, 238], and nut allergies [239] with either the hook worm N.americanus or the whipworm T.suis have failed to demonstrate any definitive results. Although, it should be noted that several studies have found success in the use of helminthic therapy for the ongoing treatment of patients suffering from ulcerative colitis [240], indicating that there may indeed be a niche for helminthic therapy in the future of the treatment for allergic disease.
Summary
With the increased incidence of allergic diseases, a better understanding of the developmental events that leads to immune sensitization against otherwise innocuous environment antigens is a key to the development of rational intervention strategies. The hygiene hypothesis, first put forward over 30 years ago, has now been expanded to include the effects of microbial dysbiosis with the aid of next generation sequencing techniques [205], leading to a much more robust view of how early life exposure to a diverse array of microbes is important for the development of the immune system and the establishment of a homeostatic relationship with our environment [204, 241], especially at the key barrier organs associated with atopy, namely the skin, airways, and gut. The initial premise of the hygiene hypothesis, that a reduction in exposure to viral and bacterial infections during childhood was responsible for the induction of default atopic Th2 responses, has now been largely disproven [199, 201, 202]. Instead, studies demonstrating the importance of the microbiome in modulating the cohort of regulatory cells induced during development which establish a tolerogenic environment and mediate suppression of T cells that arise from inflammatory lineages [148] allows for a better explanation of the observations, that is in parallel to the emergence of hay fever as a “post-industrial revolution epidemic” [179] and the rise of the atopic march [6], that we have also seen a significant increase in the incidence of autoimmunity over the same period.
Multiple studies have described that distinct microbial species found to be associated with either the establishment of a tolerogenic or inflammatory atopic state at the barrier organs. However, the use of probiotics either in a prophylactic measure or to treat established atopic disease has proved to be largely ineffective, so far (Table 1). This may be due to a number of factors including the limited number of probiotics used, the lack of specific targeting of probiotics to individual patients, or the inability of the administered probiotic to reach the specific intestinal niche that would be aided by more precise targeting. Fecal transfer therapies especially those administered by colonoscopy have had great success [215], potentially due to the diverse range of bacteria being transferred from healthy individuals, in addition to avoidance of the stomach environment and enabling access to a broader region of the intestinal system. However, it should be noted that bacteriotherapy systems for oral administration are also being developed and are proving to be effective [242]. Studies are currently underway to assess whether these means of treatment will be effective for atopic disease and the results are eagerly anticipated [216]. An additional avenue which may prove highly useful for future therapy is through the engineering of specific functions into agents for bacteriotherapy. An example of this approach was recently demonstrated in an animal model, where a strain of Bacteroides engineered to produce the secondary bile acid isoDCA was introduced and shown to be a potent inducer of gut-associated pTreg cells [167], which can dampen the immune response and support the metabolic function of the gut microbiota [243]. Together, the combined study of the microbiome and the reciprocal relationship of microbes with the development of the immune system has the broad potential for a better understanding of human health and to increase treatment options for atopic diseases, with the hygiene hypothesis proving integral to this path, by shining an initial light on how the microbial dysbiosis prevalent in industrialized societies has affected the regulation of the tolerance inducing mechanisms required for the maintenance of a homeostatic equilibrium with our external environments.
References
Draaisma E et al (2015) A multinational study to compare prevalence of atopic dermatitis in the first year of life. Pediatr Allergy Immunol 26(4):359–366
Mallol J et al (2016) Prevalence, severity, and treatment of recurrent wheezing during the first year of life: a cross-sectional study of 12,405 Latin American infants. Allergy Asthma Immunol Res 8(1):22–31
Osborne NJ et al (2011) Prevalence of challenge-proven IgE-mediated food allergy using population-based sampling and predetermined challenge criteria in infants. J Allergy Clin Immunol 127(3):668–76, e1–2
Leung ASY, Wong GWK, Tang MLK (2018) Food allergy in the developing world. J Allergy Clin Immunol 141(1):76–78, e1
Alduraywish SA et al (2016) The march from early life food sensitization to allergic disease: a systematic review and meta-analyses of birth cohort studies. Allergy 71(1):77–89
Thomsen SF (2015) Epidemiology and natural history of atopic diseases. Eur Clin Respir J 2
Hill DA, Spergel JM (2018) The atopic march: critical evidence and clinical relevance. Ann Allergy Asthma Immunol 120(2):131–137
Litonjua AA et al (1998) Parental history and the risk for childhood asthma. Does mother confer more risk than father? Am J Respir Crit Care Med 158(1):176–81
Backhed F et al (2015) Dynamics and stabilization of the human gut microbiome during the first year of life. Cell Host Microbe 17(5):690–703
Heine RG (2018) Food allergy prevention and treatment by targeted nutrition. Ann Nutr Metab 72(Suppl 3):33–45
Oddy WH (2017) Breastfeeding, childhood asthma, and allergic disease. Ann Nutr Metab 70(Suppl 2):26–36
Wang S et al (2022) Association between breastmilk microbiota and food allergy in infants. Front Cell Infect Microbiol 11
Grieger JA et al (2016) In utero programming of allergic susceptibility. Int Arch Allergy Immunol 169(2):80–92
Martino D, Prescott S (2011) Epigenetics and prenatal influences on asthma and allergic airways disease. Chest 139(3):640–647
Yassour M et al (2016) Natural history of the infant gut microbiome and impact of antibiotic treatment on bacterial strain diversity and stability. Sci Transl Med 8(343):343ra81
Slob EMA et al (2020) Early-life antibiotic use and risk of asthma and eczema: results of a discordant twin study. Eur Respir J 55(4)
Pitter G et al (2016) Antibiotic exposure in the first year of life and later treated asthma, a population based birth cohort study of 143,000 children. Eur J Epidemiol 31(1):85–94
Biasucci G et al (2010) Mode of delivery affects the bacterial community in the newborn gut. Early Hum Dev 86(Suppl 1):13–15
Lin J et al (2021) The associations of caesarean delivery with risk of wheezing diseases and changes of T Cells in children. Front Immunol 12:793762
Juhn YJ et al (2005) Mode of delivery at birth and development of asthma: a population-based cohort study. J Allergy Clin Immunol 116(3):510–516
Kumar M et al (2020) Microbiome as an immunological modifier. Methods Mol Biol 2055:595–638
Guerin LR, Prins JR, Robertson SA (2009) Regulatory T-cells and immune tolerance in pregnancy: a new target for infertility treatment? Hum Reprod Update 15(5):517–535
Soyer OU et al (2013) Mechanisms of peripheral tolerance to allergens. Allergy 68(2):161–170
Daley D (2014) The evolution of the hygiene hypothesis: the role of early-life exposures to viruses and microbes and their relationship to asthma and allergic diseases. Curr Opin Allergy Clin Immunol 14(5):390–396
von Mutius E, Vercelli D (2010) Farm living: effects on childhood asthma and allergy. Nat Rev Immunol 10(12):861–868
Genuneit J et al (2013) The combined effects of family size and farm exposure on childhood hay fever and atopy. Pediatr Allergy Immunol 24(3):293–298
Kumar M, Garand M, Al Khodor S (2019) Integrating omics for a better understanding of inflammatory bowel disease: a step towards personalized medicine. J Transl Med 17(1):419
Douwes J et al (2008) Farm exposure in utero may protect against asthma, hay fever and eczema. Eur Respir J 32(3):603–611
Ege MJ et al (2011) Exposure to environmental microorganisms and childhood asthma. N Engl J Med 364(8):701–709
Beckhaus AA et al (2015) Maternal nutrition during pregnancy and risk of asthma, wheeze, and atopic diseases during childhood: a systematic review and meta-analysis. Allergy 70(12):1588–1604
Calzada D et al (2018) Immunological mechanisms in allergic diseases and allergen tolerance: the role of treg cells. J Immunol Res 2018:6012053
Fujimura KE et al (2016) Neonatal gut microbiota associates with childhood multisensitized atopy and T cell differentiation. Nat Med 22:1187
Penders J et al (2007) Gut microbiota composition and development of atopic manifestations in infancy: the KOALA Birth Cohort Study. Gut 56(5):661–667
Paller AS et al (2019) The microbiome in patients with atopic dermatitis. J Allergy Clin Immunol 143(1):26–35
Honda K, Littman DR (2016) The microbiota in adaptive immune homeostasis and disease. Nature 535(7610):75–84
Weidinger S et al (2018) Atopic dermatitis. Nat Rev Dis Primers 4(1):1
Nutten S (2015) Atopic dermatitis: global epidemiology and risk factors. Ann Nutr Metab 66(Suppl 1):8–16
Barbarot S et al (2018) Epidemiology of atopic dermatitis in adults: results from an international survey. Allergy 73(6):1284–1293
Knor T, Meholjić-Fetahović A, Mehmedagić A (2011) Stratum corneum hydration and skin surface pH in patients with atopic dermatitis. Acta Dermatovenerol Croat 19(4):242–247
Vu AT et al (2010) Staphylococcus aureus membrane and diacylated lipopeptide induce thymic stromal lymphopoietin in keratinocytes through the Toll-like receptor 2-Toll-like receptor 6 pathway. J Allergy Clin Immunol 126(5):985–93, 993.e1–3
Naik S et al (2015) Commensal-dendritic-cell interaction specifies a unique protective skin immune signature. Nature 520(7545):104–108
Ridaura VK et al (2018) Contextual control of skin immunity and inflammation by Corynebacterium. J Exp Med 215(3):785–799
Kong HH et al (2012) Temporal shifts in the skin microbiome associated with disease flares and treatment in children with atopic dermatitis. Genome Res 22(5):850–859
Baker BS (2006) The role of microorganisms in atopic dermatitis. Clin Exp Immunol 144(1):1–9
Chng KR et al (2016) Whole metagenome profiling reveals skin microbiome-dependent susceptibility to atopic dermatitis flare. Nat Microbiol 1(9):16106
Byrd AL et al (2017) Staphylococcus aureus and Staphylococcus epidermidis strain diversity underlying pediatric atopic dermatitis. Sci Transl Med 9(397)
Simpson EL et al (2018) Patients with atopic dermatitis colonized with Staphylococcus aureus have a distinct phenotype and endotype. J Investig Dermatol 138(10):2224–2233
Watanabe S et al (2003) Differences in fecal microflora between patients with atopic dermatitis and healthy control subjects. J Allergy Clin Immunol 111(3):587–591
Kalliomäki M et al (2001) Distinct patterns of neonatal gut microflora in infants in whom atopy was and was not developing. J Allergy Clin Immunol 107(1):129–134
Lyons A et al (2010) Bacterial strain-specific induction of Foxp3+ T regulatory cells is protective in murine allergy models. Clin Exp Allergy 40(5):811–819
Gehring U et al (2001) Exposure to endotoxin decreases the risk of atopic eczema in infancy: a cohort study. J Allergy Clin Immunol 108(5):847–854
Mazmanian SK, Round JL, Kasper DL (2008) A microbial symbiosis factor prevents intestinal inflammatory disease. Nature 453(7195):620–625
Round JL, Mazmanian SK (2010) Inducible Foxp3+ regulatory T-cell development by a commensal bacterium of the intestinal microbiota. Proc Natl Acad Sci U S A 107(27):12204–12209
Abrahamsson TR et al (2012) Low diversity of the gut microbiota in infants with atopic eczema. J Allergy Clin Immunol 129(2):434–40, 440.e1–2
Nibbering B, Ubags NDJ (2020) Microbial interactions in the atopic march. Clin Exp Immunol 199(1):12–23
Doreswamy V, Peden DB (2011) Modulation of asthma by endotoxin. Clin Exp Allergy 41(1):9–19
Koh A et al (2016) From dietary fiber to host physiology: short-chain fatty acids as key bacterial metabolites. Cell 165(6):1332–1345
De Benedetto A et al (2011) Tight junction defects in patients with atopic dermatitis. J Allergy Clin Immunol 127(3):773–86.e1–7
Song H et al (2016) Faecalibacterium prausnitzii subspecies-level dysbiosis in the human gut microbiome underlying atopic dermatitis. J Allergy Clin Immunol 137(3):852–860
Reddel S et al (2019) Gut microbiota profile in children affected by atopic dermatitis and evaluation of intestinal persistence of a probiotic mixture. Sci Rep 9(1):4996
Furusawa Y et al (2013) Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature 504(7480):446–450
Loh W, Tang ML (2018) The epidemiology of food allergy in the global context. Int J Environ Res Public Health 15(9)
Sicherer SH, Sampson HA (2014) Food allergy: epidemiology, pathogenesis, diagnosis, and treatment. J Allergy Clin Immunol 133(2):291-307.e5
Sicherer SH, Sampson HA (2006) Food allergy. J Allergy Clin Immunol 117(2 Supplement 2):S470–S475
Yu W, Freeland DMH, Nadeau KC (2016) Food allergy: immune mechanisms, diagnosis and immunotherapy. Nat Rev Immunol 16(12):751–765
Tordesillas L, Berin MC (2018) Mechanisms of oral tolerance. Clin Rev Allergy Immunol 55(2):107–117
Tan J et al (2016) Dietary fiber and bacterial SCFA enhance oral tolerance and protect against food allergy through diverse cellular pathways. Cell Rep 15(12):2809–2824
Satitsuksanoa P et al (2018) Regulatory immune mechanisms in tolerance to food allergy. Front Immunol 9:2939
Akdis CA, Akdis M (2014) Mechanisms of immune tolerance to allergens: role of IL-10 and Tregs. J Clin Invest 124(11):4678–4680
Di Costanzo M et al (2020) Gut microbiome modulation for preventing and treating pediatric food allergies. Int J Mol Sci 21(15):5275
Paparo L et al (2021) Butyrate as a bioactive human milk protective component against food allergy. Allergy 76(5):1398–1415
Zhao W, Ho HE, Bunyavanich S (2019) The gut microbiome in food allergy. Ann Allergy Asthma Immunol 122(3):276–282
Klampfer L et al (2003) Inhibition of interferon gamma signaling by the short chain fatty acid butyrate. Mol Cancer Res 1(11):855–862
Lee WJ, Hase K (2014) Gut microbiota-generated metabolites in animal health and disease. Nat Chem Biol 10(6):416–424
Sokol H et al (2008) Faecalibacterium prausnitzii is an anti-inflammatory commensal bacterium identified by gut microbiota analysis of Crohn disease patients. Proc Natl Acad Sci U S A 105(43):16731–16736
Han KJ et al (2015) Anticancer and anti-inflammatory activity of probiotic Lactococcus lactis NK34. J Microbiol Biotechnol 25(10):1697–1701
Azad MB et al (2015) Infant gut microbiota and food sensitization: associations in the first year of life. Clin Exp Allergy 45(3):632–643
Savage JH et al (2018) A prospective microbiome-wide association study of food sensitization and food allergy in early childhood. Allergy 73(1):145–152
Yatsunenko T et al (2012) Human gut microbiome viewed across age and geography. Nature 486(7402):222–227
McCoy KD, Thomson CA (2018) The impact of maternal microbes and microbial colonization in early life on hematopoiesis. J Immunol 200(8):2519–2526
Kubinak JL et al (2015) MyD88 signaling in T cells directs IgA-mediated control of the microbiota to promote health. Cell Host Microbe 17(2):153–163
Abdel-Gadir A et al (2019) Microbiota therapy acts via a regulatory T cell MyD88/RORγt pathway to suppress food allergy. Nat Med 25(7):1164–1174
Hufnagl K et al (2020) Dysbiosis of the gut and lung microbiome has a role in asthma. Seminars in Immunopathology 42(1):75–93
Stein RT et al (1999) Respiratory syncytial virus in early life and risk of wheeze and allergy by age 13 years. Lancet 354(9178):541–545
Arrieta MC et al (2015) Early infancy microbial and metabolic alterations affect risk of childhood asthma. Sci Transl Med 7(307):307ra152
Riedler J et al (2001) Exposure to farming in early life and development of asthma and allergy: a cross-sectional survey. Lancet 358(9288):1129–1133
Hyytiäinen H et al (2021) Microbial diversity in homes and the risk of allergic rhinitis and inhalant atopy in two European birth cohorts. Environ Res 196:110835
Singleton TE, Massari P, Wetzler LM (2005) Neisserial porin-induced dendritic cell activation is MyD88 and TLR2 dependent. J Immunol 174(6):3545–3550
Meyer EH et al (2006) Glycolipid activation of invariant T cell receptor+ NK T cells is sufficient to induce airway hyperreactivity independent of conventional CD4+ T cells. Proc Natl Acad Sci U S A 103(8):2782–2787
Larsen JM et al (2015) Chronic obstructive pulmonary disease and asthma-associated Proteobacteria, but not commensal Prevotella spp., promote Toll-like receptor 2-independent lung inflammation and pathology. Immunology 144(2):333–42
Naleway AL (2004) Asthma and atopy in rural children: is farming protective? Clin Med Res 2(1):5–12
von Mutius E, Vercelli D (2010) Farm living: effects on childhood asthma and allergy. Nat Rev Immunol 10:861
Wlasiuk G, Vercelli D (2012) The farm effect, or: when, what and how a farming environment protects from asthma and allergic disease. Curr Opin Allergy Clin Immunol 12(5):461–466
Schuijs MJ et al (2015) Farm dust and endotoxin protect against allergy through A20 induction in lung epithelial cells. Science 349(6252):1106–1110
Kakli HA, Riley TD (2016) Allergic rhinitis. Prim Care 43(3):465–475
Wheatley LM, Togias A (2015) Clinical practice. Allergic rhinitis N Engl J Med 372(5):456–463
Chiu CY et al (2017) Airway microbial diversity is inversely associated with mite-sensitized rhinitis and asthma in early childhood. Sci Rep 7(1):1820
Yu W et al (2015) Reduced airway microbiota diversity is associated with elevated allergic respiratory inflammation. Ann Allergy Asthma Immunol 115(1):63–68
Marri PR et al (2013) Asthma-associated differences in microbial composition of induced sputum. J Allergy Clin Immunol 131(2):346-352.e3
Huang YJ, Boushey HA (2015) The microbiome in asthma. J Allergy Clin Immunol 135(1):25–30
Sverrild A et al (2017) Eosinophilic airway inflammation in asthmatic patients is associated with an altered airway microbiome. J Allergy Clin Immunol 140(2):407-417.e11
Green BJ et al (2014) Potentially pathogenic airway bacteria and neutrophilic inflammation in treatment resistant severe asthma. Plos One 9(6):e100645
Taylor SL et al (2018) Inflammatory phenotypes in patients with severe asthma are associated with distinct airway microbiology. J Allergy Clin Immunol 141(1):94-103.e15
Goleva E et al (2013) The effects of airway microbiome on corticosteroid responsiveness in asthma. Am J Respir Crit Care Med 188(10):1193–1201
Abrahamsson TR et al (2014) Low gut microbiota diversity in early infancy precedes asthma at school age. Clin Exp Allergy 44(6):842–850
Stiemsma LT et al (2016) Shifts in Lachnospira and Clostridium sp. in the 3-month stool microbiome are associated with preschool age asthma. Clin Sci 130(23):2199–2207
Durack J et al (2018) Delayed gut microbiota development in high-risk for asthma infants is temporarily modifiable by Lactobacillus supplementation. Nat Commun 9(1):707
Jimenez E et al (2005) Isolation of commensal bacteria from umbilical cord blood of healthy neonates born by cesarean section. Curr Microbiol 51(4):270–274
Stout MJ et al (2013) Identification of intracellular bacteria in the basal plate of the human placenta in term and preterm gestations. Am J Obstet Gynecol 208(3):226, e1–7
Aagaard K et al (2014) The placenta harbors a unique microbiome. Sci Transl Med 6(237):237ra65
Al Alam D et al (2020) Human fetal lungs harbor a microbiome signature. Am J Respir Crit Care Med 201(8):1002–1006
Rackaityte E et al (2020) Viable bacterial colonization is highly limited in the human intestine in utero. Nat Med 26(4):599–607
Senn V et al (2020) Microbial colonization from the fetus to early childhood - a comprehensive review. Front Cell Infect Microbiol 10:573735
Stinson LF et al (2019) The Not-so-sterile womb: evidence that the human fetus is exposed to bacteria prior to birth. Front Microbiol 10:1124
Blaser MJ et al (2021) Lessons learned from the prenatal microbiome controversy. Microbiome 9(1):8
Walter J, Hornef MW (2021) A philosophical perspective on the prenatal in utero microbiome debate. Microbiome 9(1):5
Mishra A et al (2021) Microbial exposure during early human development primes fetal immune cells. Cell 184(13):3394–3409, e20
Prince AL et al (2015) The perinatal microbiome and pregnancy: moving beyond the vaginal microbiome. Cold Spring Harb Perspect Med 5(6)
Wampach L et al (2018) Birth mode is associated with earliest strain-conferred gut microbiome functions and immunostimulatory potential. Nat Commun 9(1):5091
Ferretti P et al (2018) Mother-to-infant microbial transmission from different body sites shapes the developing infant gut microbiome. Cell Host Microbe 24(1):133–145, e5
Sordillo JE et al (2010) Multiple microbial exposures in the home may protect against asthma or allergy in childhood. Clin Exp Allergy 40(6):902–910
Gensollen T et al (2016) How colonization by microbiota in early life shapes the immune system. Science 352(6285):539–544
Keag OE, Norman JE, Stock SJ (2018) Long-term risks and benefits associated with cesarean delivery for mother, baby, and subsequent pregnancies: systematic review and meta-analysis. PLoS Med 15(1):e1002494
Shao Y et al (2019) Stunted microbiota and opportunistic pathogen colonization in caesarean-section birth. Nature 574(7776):117–121
Dominguez-Bello MG et al (2016) Partial restoration of the microbiota of cesarean-born infants via vaginal microbial transfer. Nat Med 22(3):250–253
Bezirtzoglou E, Tsiotsias A, Welling GW (2011) Microbiota profile in feces of breast- and formula-fed newborns by using fluorescence in situ hybridization (FISH). Anaerobe 17(6):478–482
Tilburgs T, Scherjon SA, Claas FH (2010) Major histocompatibility complex (MHC)-mediated immune regulation of decidual leukocytes at the fetal-maternal interface. J Reprod Immunol 85(1):58–62
Schreurs R et al (2019) Human fetal TNF-α-cytokine-producing CD4(+) effector memory T cells promote intestinal development and mediate inflammation early in life. Immunity 50(2):462-476.e8
Blom B, Res PC, Spits H (1998) T cell precursors in man and mice. Crit Rev Immunol 18(4):371–388
Haynes BF, Heinly CS (1995) Early human T cell development: analysis of the human thymus at the time of initial entry of hematopoietic stem cells into the fetal thymic microenvironment. J Exp Med 181(4):1445–1458
Park JE et al (2020) Prenatal development of human immunity. Science 368(6491):600–603
Vuillermin PJ et al (2017) The maternal microbiome during pregnancy and allergic disease in the offspring. Semin Immunopathol 39(6):669–675
Stokholm J et al (2017) Preeclampsia associates with asthma, allergy, and eczema in childhood. Am J Respir Crit Care Med 195(5):614–621
Michaëlsson J et al (2006) Regulation of T cell responses in the developing human fetus. J Immunol 176(10):5741–5748
Mold JE et al (2008) Maternal alloantigens promote the development of tolerogenic fetal regulatory T cells in utero. Science 322(5907):1562–1565
La Rocca C et al (2014) The immunology of pregnancy: regulatory T cells control maternal immune tolerance toward the fetus. Immunol Lett 162(1 Pt A):41–8
Kumar M et al (2021) Vaginal microbiota and cytokine levels predict preterm delivery in Asian women. Front Cell Infect Microbiol 11:639665
McGovern N et al (2017) Human fetal dendritic cells promote prenatal T-cell immune suppression through arginase-2. Nature 546(7660):662–666
Basha S, Surendran N, Pichichero M (2014) Immune responses in neonates. Expert Rev Clin Immunol 10(9):1171–1184
Cosmi L et al (2008) Human interleukin 17-producing cells originate from a CD161+CD4+ T cell precursor. J Exp Med 205(8):1903–1916
Debock I, Flamand V (2014) Unbalanced neonatal cD4(+) T-cell immunity. Front Immunol 5:393
Peterson J et al (2009) The NIH human microbiome project. Genome Res 19(12):2317–2323
Lee N, Kim WU (2017) Microbiota in T-cell homeostasis and inflammatory diseases. Exp Mol Med 49(5):e340
Yang BH et al (2016) Foxp3(+) T cells expressing RORgammat represent a stable regulatory T-cell effector lineage with enhanced suppressive capacity during intestinal inflammation. Mucosal Immunol 9(2):444–457
Lathrop SK et al (2011) Peripheral education of the immune system by colonic commensal microbiota. Nature 478(7368):250–254
Kim KS et al (2016) Dietary antigens limit mucosal immunity by inducing regulatory T cells in the small intestine. Science 351(6275):858–863
Bacher P et al (2016) Regulatory T cell specificity directs tolerance versus allergy against aeroantigens in humans. Cell 167(4):1067-1078.e16
Bilate AM, Lafaille JJ (2012) Induced CD4+Foxp3+ regulatory T cells in immune tolerance. Annu Rev Immunol 30:733–758
Kim SV et al (2013) GPR15-mediated homing controls immune homeostasis in the large intestine mucosa. Science 340(6139):1456–1459
Bacher P, Scheffold A (2018) Antigen-specific regulatory T-cell responses against aeroantigens and their role in allergy. Mucosal Immunol
Shamji MH, Durham SR (2017) Mechanisms of allergen immunotherapy for inhaled allergens and predictive biomarkers. J Allergy Clin Immunol 140(6):1485–1498
Wambre E et al (2017) A phenotypically and functionally distinct human TH2 cell subpopulation is associated with allergic disorders. Sci Transl Med 9(401)
Bangert C et al (2021) Persistence of mature dendritic cells, TH2A, and Tc2 cells characterize clinically resolved atopic dermatitis under IL-4Ralpha blockade. Sci Immunol 6(55)
Zhu J, Yamane H, Paul WE (2010) Differentiation of effector CD4 T cell populations (*). Annu Rev Immunol 28:445–489
Roychoudhuri R et al (2013) BACH2 represses effector programs to stabilize T(reg)-mediated immune homeostasis. Nature 498(7455):506–510
Herbst T et al (2011) Dysregulation of allergic airway inflammation in the absence of microbial colonization. Am J Respir Crit Care Med 184(2):198–205
McCoy KD et al (2006) Natural IgE production in the absence of MHC Class II cognate help. Immunity 24(3):329–339
Josefowicz SZ et al (2012) Extrathymically generated regulatory T cells control mucosal TH2 inflammation. Nature 482(7385):395–399
Belkaid Y, Hand TW (2014) Role of the microbiota in immunity and inflammation. Cell 157(1):121–141
Ohnmacht C et al (2015) Mucosal immunology. The microbiota regulates type 2 immunity through RORgammat(+) T cells. Science 349(6251):989–93
Russell SL et al (2012) Early life antibiotic-driven changes in microbiota enhance susceptibility to allergic asthma. EMBO Rep 13(5):440–447
Bhaskaran N et al (2018) Role of short chain fatty acids in controlling tregs and immunopathology during mucosal infection. Front Microbiol 9:1995
Smith PM et al (2013) The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science 341(6145):569–573
Thio CL et al (2018) Regulation of type 2 innate lymphoid cell-dependent airway hyperreactivity by butyrate. J Allergy Clin Immunol 142(6):1867–1883, e12
Pandiyan P et al (2019) Microbiome dependent regulation of tregs and Th17 Cells in mucosa. Front Immunol 10:426
Ridlon JM et al (2016) Consequences of bile salt biotransformations by intestinal bacteria. Gut Microbes 7(1):22–39
Campbell C et al (2020) Bacterial metabolism of bile acids promotes generation of peripheral regulatory T cells. Nature 581(7809):475–479
Fujimura KE et al (2016) Neonatal gut microbiota associates with childhood multisensitized atopy and T cell differentiation. Nat Med 22(10):1187–1191
Levan SR et al (2019) Elevated faecal 12,13-diHOME concentration in neonates at high risk for asthma is produced by gut bacteria and impedes immune tolerance. Nat Microbiol 4(11):1851–1861
Round JL et al (2011) The toll-like receptor 2 pathway establishes colonization by a commensal of the human microbiota. Science 332(6032):974–977
Verma R et al (2018) Cell surface polysaccharides of bifidobacterium bifidum induce the generation of Foxp3(+) regulatory T cells. Sci Immunol 3(28)
Kunisawa J et al (2012) A pivotal role of vitamin B9 in the maintenance of regulatory T cells in vitro and in vivo. PLoS One 7(2):e32094
Singh N et al (2014) Activation of Gpr109a, receptor for niacin and the commensal metabolite butyrate, suppresses colonic inflammation and carcinogenesis. Immunity 40(1):128–139
Wang Y et al (2021) Interaction of maternal asthma history and plasma folate levels on child asthma risk in the Boston Birth Cohort. Pediatr Pulmonol 56(12):3728–3736
McGowan EC et al (2020) Association between folate metabolites and the development of food allergy in children. J Allergy Clin Immunol Pract 8(1):132–140, e5
Karimi K et al (2009) Lactobacillus reuteri-induced regulatory T cells protect against an allergic airway response in mice. Am J Respir Crit Care Med 179(3):186–193
Cervantes-Barragan L et al (2017) Lactobacillus reuteri induces gut intraepithelial CD4(+)CD8alphaalpha(+) T cells. Science 357(6353):806–810
Henrick BM et al (2021) Bifidobacteria-mediated immune system imprinting early in life. Cell 184(15):3884–3898, e11
Strachan DP (1989) Hay fever, hygiene, and household size. BMJ 299(6710):1259–1260
Shirakawa T et al (1997) The inverse association between tuberculin responses and atopic disorder. Science 275(5296):77–79
Illi S et al (2001) Early childhood infectious diseases and the development of asthma up to school age: a birth cohort study. BMJ 322(7283):390–395
Figueiredo CA et al (2013) Environmental conditions, immunologic phenotypes, atopy, and asthma: new evidence of how the hygiene hypothesis operates in Latin America. J Allergy Clin Immunol 131(4):1064–1068, e1
Silverberg JI et al (2010) Association between varicella zoster virus infection and atopic dermatitis in early and late childhood: a case-control study. J Allergy Clin Immunol 126(2):300–305
Bremner SA et al (2008) Infections presenting for clinical care in early life and later risk of hay fever in two UK birth cohorts. Allergy 63(3):274–283
Benn CS et al (2004) Cohort study of sibling effect, infectious diseases, and risk of atopic dermatitis during first 18 months of life. BMJ 328(7450):1223
Dunder T et al (2007) Infections in child day care centers and later development of asthma, allergic rhinitis, and atopic dermatitis: prospective follow-up survey 12 years after controlled randomized hygiene intervention. Arch Pediatr Adolesc Med 161(10):972–977
Bach JF (2002) The effect of infections on susceptibility to autoimmune and allergic diseases. N Engl J Med 347(12):911–920
Steinman L (2007) A brief history of T(H)17, the first major revision in the T(H)1/T(H)2 hypothesis of T cell-mediated tissue damage. Nat Med 13(2):139–145
Simpson CR et al (2002) Coincidence of immune-mediated diseases driven by Th1 and Th2 subsets suggests a common aetiology. A population-based study using computerized general practice data. Clin Exp Allergy 32(1):37–42
Smew AI et al (2020) Familial coaggregation of asthma and type 1 diabetes in children. JAMA Netw Open 3(3):e200834
Guo R et al (2017) Atopy in children with juvenile systemic lupus erythematosus is associated with severe disease. PLoS One 12(5):e0177774
Krishna MT et al (2019) Allergic diseases and long-term risk of autoimmune disorders: longitudinal cohort study and cluster analysis. Eur Respir J 54(5)
Kreiner E et al (2017) Shared genetic variants suggest common pathways in allergy and autoimmune diseases. J Allergy Clin Immunol 140(3):771–781
Schabussova I, Wiedermann U (2014) Allergy and worms: let’s bring back old friends? Wien Med Wochenschr 164(19–20):382–391
Elliott AM et al (2005) Helminth infection during pregnancy and development of infantile eczema. JAMA 294(16):2032–2034
Cooper PJ et al (2018) Effect of early-life geohelminth infections on the development of wheezing at 5 years of age. Am J Respir Crit Care Med 197(3):364–372
Endara P et al (2010) Long-term periodic anthelmintic treatments are associated with increased allergen skin reactivity. Clin Exp Allergy 40(11):1669–1677
Leonardi-Bee J, Pritchard D, Britton J (2006) Asthma and current intestinal parasite infection: systematic review and meta-analysis. Am J Respir Crit Care Med 174(5):514–523
Alexandre-Silva GM et al (2018) The hygiene hypothesis at a glance: early exposures, immune mechanism and novel therapies. Acta Trop 188:16–26
Araujo MI et al (2000) Inverse association between skin response to aeroallergens and Schistosoma mansoni infection. Int Arch Allergy Immunol 123(2):145–148
van Tilburg Bernardes E, Arrieta MC (2017) Hygiene hypothesis in asthma development: is hygiene to blame? Arch Med Res 48(8):717–726
Bloomfield SF et al (2016) Time to abandon the hygiene hypothesis: new perspectives on allergic disease, the human microbiome, infectious disease prevention and the role of targeted hygiene. Perspect Public Health 136(4):213–224
Rook GA, Martinelli R, Brunet LR (2003) Innate immune responses to mycobacteria and the downregulation of atopic responses. Curr Opin Allergy Clin Immunol 3(5):337–342
Haahtela T et al (2013) The biodiversity hypothesis and allergic disease: world allergy organization position statement. World Allergy Organ J 6(1):3
Lambrecht BN, Hammad H (2017) The immunology of the allergy epidemic and the hygiene hypothesis. Nat Immunol 18(10):1076–1083
Parfrey LW et al (2014) Communities of microbial eukaryotes in the mammalian gut within the context of environmental eukaryotic diversity. Front Microbiol 5:298
Venkatakrishnan A et al (2021) Evolution of bacteria in the human gut in response to changing environments: an invisible player in the game of health. Comput Struct Biotechnol J 19:752–758
Sonnenburg ED et al (2016) Diet-induced extinctions in the gut microbiota compound over generations. Nature 529(7585):212–215
Byndloss MX et al (2017) Microbiota-activated PPAR-gamma signaling inhibits dysbiotic Enterobacteriaceae expansion. Science 357(6351):570–575
Foegeding NJ, Jones ZS, Byndloss MX (2021) Western lifestyle as a driver of dysbiosis in colorectal cancer. Dis Model Mech 14(5)
Smits SA et al (2017) Seasonal cycling in the gut microbiome of the Hadza hunter-gatherers of Tanzania. Science 357(6353):802–806
Tyakht AV et al (2013) Human gut microbiota community structures in urban and rural populations in Russia. Nat Commun 4:2469
Fan Y, Pedersen O (2021) Gut microbiota in human metabolic health and disease. Nat Rev Microbiol 19(1):55–71
Komlosi ZI et al (2021) Cellular and molecular mechanisms of allergic asthma. Mol Aspects Med 100995
Kelly CR et al (2014) Fecal microbiota transplant for treatment of Clostridium difficile infection in immunocompromised patients. Am J Gastroenterol 109(7):1065–1071
NCT02960074 (2016) https://clinicaltrials.gov/ct2/show/NCT02960074. Accessed 24 April 2022
Feehley T et al (2019) Healthy infants harbor intestinal bacteria that protect against food allergy. Nat Med 25(3):448–453
Myles IA et al (2018) First-in-human topical microbiome transplantation with Roseomonas mucosa for atopic dermatitis. JCI Insight 3(9)
Nakatsuji T et al (2021) Development of a human skin commensal microbe for bacteriotherapy of atopic dermatitis and use in a phase 1 randomized clinical trial. Nat Med 27(4):700–709
Tang ML et al (2015) Administration of a probiotic with peanut oral immunotherapy: a randomized trial. J Allergy Clin Immunol 135(3):737–44 e8
Hol J et al (2008) The acquisition of tolerance toward cow’s milk through probiotic supplementation: a randomized, controlled trial. J Allergy Clin Immunol 121(6):1448–1454
Berni Canani R et al (2012) Effect of Lactobacillus GG on tolerance acquisition in infants with cow’s milk allergy: a randomized trial. J Allergy Clin Immunol 129(2):580–2, 582 e1–5
Candy DCA et al (2018) A synbiotic-containing amino-acid-based formula improves gut microbiota in non-IgE-mediated allergic infants. Pediatr Res 83(3):677–686
Wickens K et al (2012) A protective effect of Lactobacillus rhamnosus HN001 against eczema in the first 2 years of life persists to age 4 years. Clin Exp Allergy 42(7):1071–1079
Boyle RJ et al (2011) Lactobacillus GG treatment during pregnancy for the prevention of eczema: a randomized controlled trial. Allergy 66(4):509–516
Kalliomaki M et al (2001) Probiotics in primary prevention of atopic disease: a randomised placebo-controlled trial. Lancet 357(9262):1076–1079
Dotterud CK et al (2010) Probiotics in pregnant women to prevent allergic disease: a randomized, double-blind trial. Br J Dermatol 163(3):616–623
Peldan P et al (2017) Perinatal probiotics decreased eczema up to 10 years of age, but at 5–10 years, allergic rhino-conjunctivitis was increased. Clin Exp Allergy 47(7):975–979
Azad MB et al (2013) Probiotic supplementation during pregnancy or infancy for the prevention of asthma and wheeze: systematic review and meta-analysis. BMJ 347:f6471
Zuccotti G et al (2015) Probiotics for prevention of atopic diseases in infants: systematic review and meta-analysis. Allergy 70(11):1356–1371
Wei X et al (2020) Association between probiotic supplementation and asthma incidence in infants: a meta-analysis of randomized controlled trials. J Asthma 57(2):167–178
Fiocchi A et al (2015) World allergy organization-mcmaster university guidelines for allergic disease prevention (GLAD-P): probiotics. World Allergy Organ J 8(1):4
Maizels RM (2020) Regulation of immunity and allergy by helminth parasites. Allergy 75(3):524–534
Rodrigues LC et al (2008) Early infection with Trichuris trichiura and allergen skin test reactivity in later childhood. Clin Exp Allergy 38(11):1769–1777
Scientific America (2010) https://www.scientificamerican.com/article/helminthic-therapy-mucus/. Accessed 24 April 2022
Feary JR et al (2010) Experimental hookworm infection: a randomized placebo-controlled trial in asthma. Clin Exp Allergy 40(2):299–306
Feary J et al (2009) Safety of hookworm infection in individuals with measurable airway responsiveness: a randomized placebo-controlled feasibility study. Clin Exp Allergy 39(7):1060–1068
Bager P et al (2010) Trichuris suis ova therapy for allergic rhinitis: a randomized, double-blind, placebo-controlled clinical trial. J Allergy Clin Immunol 125(1):123–30 e1–3
Jouvin MH, Kinet JP (2012) Trichuris suis ova: testing a helminth-based therapy as an extension of the hygiene hypothesis. J Allergy Clin Immunol 130(1):3–10; quiz 11–2
Summers RW et al (2005) Trichuris suis therapy in Crohn’s disease. Gut 54(1):87–90
Kumar M et al (2021) Omouma: a prospective mother and child cohort aiming to identify early biomarkers of pregnancy complications in women living in Qatar. BMC Pregnancy Childbirth 21(1):570
Kao D et al (2017) Effect of oral capsule- vs colonoscopy-delivered fecal microbiota transplantation on recurrent clostridium difficile infection: a randomized clinical trial. JAMA 318(20):1985–1993
Campbell C et al (2018) Extrathymically generated regulatory T Cells establish a niche for intestinal border-dwelling bacteria and affect physiologic metabolite balance. Immunity 48(6):1245–1257, e9
Acknowledgements
The authors would like to thank Paul D. Thacker for his valuable support during the preparation of this manuscript. Images used in production of figures were provided by Vecteezy.com under their free license agreement.
Funding
Open Access funding provided by the Qatar National Library. This project is financially supported by funds from Sidra Medicine to Dr. Souhaila Al Khodor and to Dr. Nicholas van Panhuys. The funding bodies played no role in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript.
Author information
Authors and Affiliations
Contributions
All the authors have drafted the manuscript and revised and approved the final version.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Augustine, T., Kumar, M., Al Khodor, S. et al. Microbial Dysbiosis Tunes the Immune Response Towards Allergic Disease Outcomes. Clinic Rev Allerg Immunol 65, 43–71 (2023). https://doi.org/10.1007/s12016-022-08939-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12016-022-08939-9