Acta Chimica Sinica ›› 2022, Vol. 80 ›› Issue (2): 150-158.DOI: 10.6023/A21100477 Previous Articles Next Articles
Article
黄擎a,b, 丁瑞a,b, 陈来a,b,*( ), 卢赟a,b, 石奇a,b, 张其雨a,b, 聂启军a,b, 苏岳锋a,b,*(
), 卢赟a,b, 石奇a,b, 张其雨a,b, 聂启军a,b, 苏岳锋a,b,*( ), 吴锋a,b
), 吴锋a,b
投稿日期:2021-10-26
发布日期:2022-01-18
通讯作者:
陈来, 苏岳锋
基金资助:
Qing Huanga,b, Rui Dinga,b, Lai Chena,b( ), Yun Lua,b, Qi Shia,b, Qiyu Zhanga,b, Qijun Niea,b, Yuefeng Sua,b(
), Yun Lua,b, Qi Shia,b, Qiyu Zhanga,b, Qijun Niea,b, Yuefeng Sua,b( ), Feng Wua,b
), Feng Wua,b
Received:2021-10-26
Published:2022-01-18
Contact:
Lai Chen, Yuefeng Su
Supported by:Share
Qing Huang, Rui Ding, Lai Chen, Yun Lu, Qi Shi, Qiyu Zhang, Qijun Nie, Yuefeng Su, Feng Wu. Dual-Decoration and Mechanism Analysis of Ni-rich LiNi0.83Co0.11Mn0.06O2 Cathodes by Na2PO3F[J]. Acta Chimica Sinica, 2022, 80(2): 150-158.

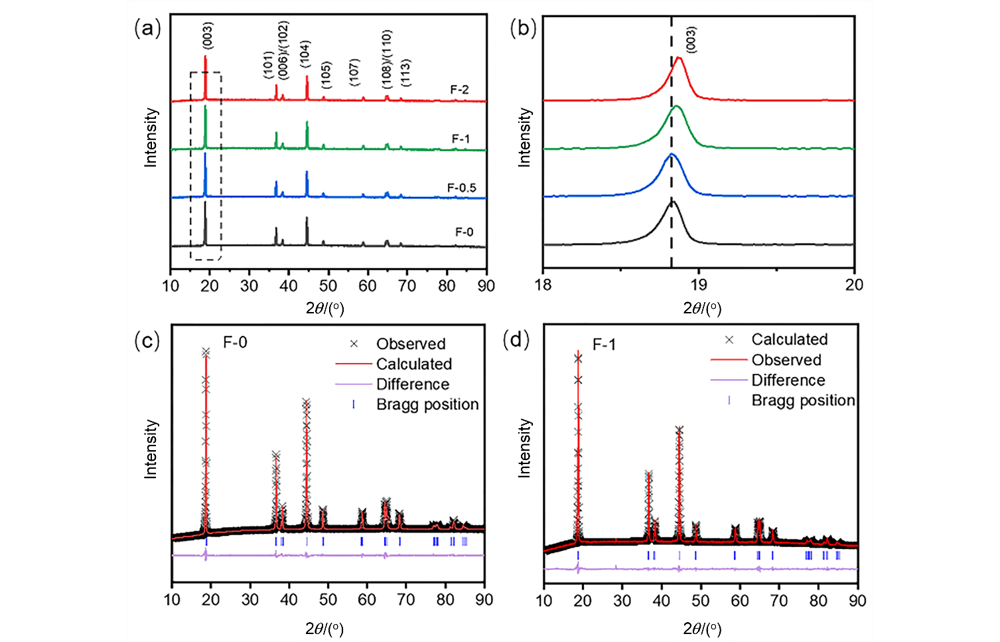
| Parameter | F-0 | F-1 |
|---|---|---|
| a/nm | 0.28699 | 0.28718 |
| c/nm | 1.41955 | 1.41949 |
| Unit volume/nm3 | 0.10125 | 0.10138 |
| c∶a | 4.946 | 4.943 |
| Rwp/% | 1.61 | 1.62 |
| Rp/% | 1.01 | 1.14 |
| Parameter | F-0 | F-1 |
|---|---|---|
| a/nm | 0.28699 | 0.28718 |
| c/nm | 1.41955 | 1.41949 |
| Unit volume/nm3 | 0.10125 | 0.10138 |
| c∶a | 4.946 | 4.943 |
| Rwp/% | 1.61 | 1.62 |
| Rp/% | 1.01 | 1.14 |

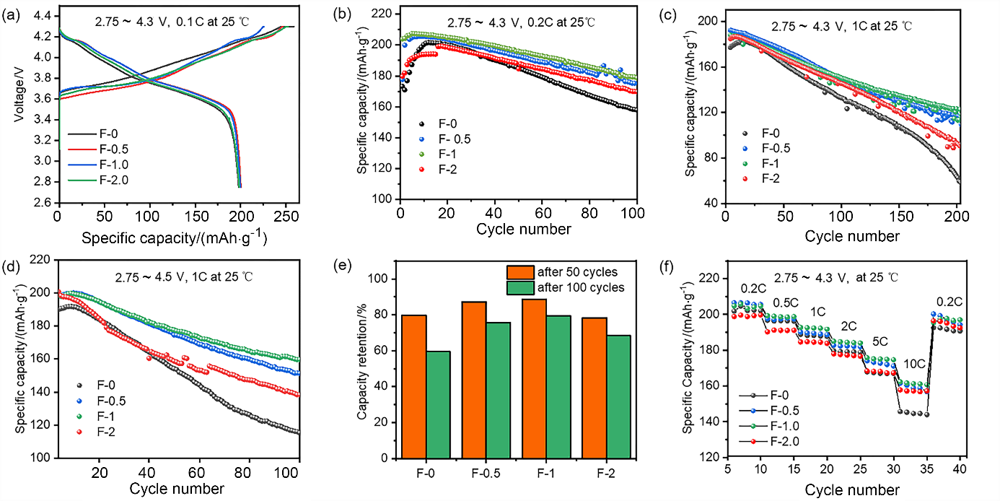
| [1] |
Liu, J.-D.; Zhang, Y.-D.; Liu, J.-X.; Li, J.-H.; Qiu, X.-G.; Cheng, F.-Y. Acta Chim. Sinica 2020, 78, 1426. (in Chinese)
doi: 10.6023/A20070330 |
|
( 刘九鼎, 张宇栋, 刘俊祥, 李金翰, 邱晓光, 程方益, 化学学报, 2020, 78, 1426.)
|
|
| [2] |
Liu, J.; Zou, Z.-G.; Zhang, S.-C.; Zhang, H.-H. J. Solid State Electrochem. 2020, 25, 387.
doi: 10.1007/s10008-020-04818-5 |
| [3] |
Su, Y.-F.; Zhang, Q.-Y.; Chen, L.; Bao, L.; Lu, Y.; Chen, S.; Wu, F. J. Energy Chem. 2022, 65, 236.
doi: 10.1016/j.jechem.2021.05.048 |
| [4] |
Manthiram, A.; Song, B.; Li, W.-D. Energy Storage Mater. 2017, 6, 125.
|
| [5] |
Li, T.-X.; Li, D.-L.; Zhang, Q.-B.; Gao, J.-H.; Kong, X.-Z.; Fan, X.-Y.; Gou, L. Acta Chim. Sinica 2021, 79, 678. (in Chinese)
doi: 10.6023/A21010019 |
|
( 李童心, 李东林, 张清波, 高建行, 孔祥泽, 樊小勇, 苟蕾, 化学学报, 2021, 79, 678.)
|
|
| [6] |
Su, Y.-F.; Li, L.-W.; Chen, G.; Chen, L.; Li, N.; Lu, Y.; Bao, L.-Y.; Chen, S.; Wu, F. Chin. J. Chem. 2020, 39, 189.
doi: 10.1002/cjoc.v39.1 |
| [7] |
Kim, J.; Lee, H.-Y.; Cha, H.-Y.; Yoon, M.-S.; Park, M.; Cho, J. Adv. Energy Mater. 2018, 8, 1702028.
doi: 10.1002/aenm.v8.6 |
| [8] |
Nam, G.-W.; Park, N.-Y.; Park, K.-J.; Yang, J.; Liu, J.; Yoon, C.-S.; Sun, Y.-K. ACS Energy Letters 2019, 4, 2995.
doi: 10.1021/acsenergylett.9b02302 |
| [9] |
Li, J.; Zhang, M.-L.; Zhang, D.-Y.; Yan, Y.-X.; Li, Z.-M. Chem. Eng. J. 2020, 402, 14.
|
| [10] |
Qiu, K.; Yan, M.-X.; Zhao, S.-W.; An, S.-L.; Wang, W.; Jia, G.-X., Acta Chim. Sinica 2021, 79, 1146. (in Chinese)
doi: 10.6023/A21040178 |
|
( 邱凯, 严铭霞, 赵守旺, 安胜利, 王玮, 贾桂霄, 化学学报, 2021, 79, 1146.)
|
|
| [11] |
Xu, H.; Ai, L.; Yan, J.-Y.; Yan, G.-L.; Zhang, W.-T. Ceram. Int. 2019, 45, 23089.
doi: 10.1016/j.ceramint.2019.08.001 |
| [12] |
Seo, J.-H.; Kim, U.-H.; Sun, Y.-K.; Yoon, C.-S. J. Electrochem. Soc. 2020, 167, 100557.
doi: 10.1149/1945-7111/ab9e82 |
| [13] |
Jia, G.-F.; Shangguan, X.-G.; Liu, S.-Q.; He, Z. Ionics 2020, 26, 4969.
doi: 10.1007/s11581-020-03683-6 |
| [14] |
Yang, R.-K.; Wu, Z.-G.; Li, Y.-C.; Li, R.; Qiu, L.; Wang, D.; Yang, L.; Guo, X.-D. Ionics 2020, 26, 3223.
doi: 10.1007/s11581-019-03399-2 |
| [15] |
Li, L.-J.; Xia, L.-F.; Yang, H.-P.; Zhan, X.-H.; Chen, J.; Chen, Z.-Y.; Duan, J.-F. J. Alloys Compd. 2020, 832, 154959.
doi: 10.1016/j.jallcom.2020.154959 |
| [16] |
Li, L.; Liu, Q.; Huang, J.-J.; Luo, S.-Y.; Sun, H.; Zheng, H.; Feng, C.-Q. J. Mater. Sci.: Mater. Electron. 2020, 31, 12409.
|
| [17] |
Jamil, S.; Yu, R.-Z.; Wang, Q.; Fasehullah, M.; Huang, Y.; Yang, Z.-H.; Yang, X.-K.; Wang, X.-Y. J. Power Sources 2020, 473, 228597.
doi: 10.1016/j.jpowsour.2020.228597 |
| [18] |
He, Y.-L.; Li, Y.; Liu, Y.; Li, W.-X.; Liu, W.-B. Mater. Chem. Phys. 2020, 251, 123085.
doi: 10.1016/j.matchemphys.2020.123085 |
| [19] |
Zhang, D.-K.; Liu, Y.; Wu, L.; Feng, L.-W.; Jin, S.-L.; Zhang, R.; Jin, M.-L. Electrochim. Acta 2019, 328, 135086.
doi: 10.1016/j.electacta.2019.135086 |
| [20] |
Liu, Z.-B.; Li, J.-G.; Zhu, M.-J.; Wang, L.; Kang, Y.-Q.; Dang, Z.-H.; Yan, J.-S.; He, X.-M. Materials 2021, 14, 1816.
doi: 10.3390/ma14081816 |
| [21] |
Wang, R.; Zhang, T.-S.; Zhang, Q.-R.; Zheng, M.-W.; Xu, K.; Yan, W.-T. Ionics 2019, 26, 1165.
doi: 10.1007/s11581-019-03316-7 |
| [22] |
Kong, F.; Liang, C.; Longo, R.-C.; Yeon, D.-H.; Zheng, Y.; Park, J.-H.; Doo, S.-G.; Cho, K. Chem. Mater. 2016, 28, 6942.
doi: 10.1021/acs.chemmater.6b02627 |
| [23] |
Vanaphuti, P.; Bai, J.-M.; Ma, L.; Ehrlich, S.; Kisslinger, K.; Wang, F.; Wang, Y. Energy Storage Mater. 2020, 31, 459.
|
| [24] |
Kim, H.; Kim, S.-B.; Park, D.-H.; Park, K.-W. Energies 2020, 13.
|
| [25] |
Qiu, Q.-Q.; Yuan, S.-S.; Bao, J.; Wang, Q.-C.; Yue, X.-Y.; Li, X.-L.; Wu, X.-J.; Zhou, Y.-N. J. Energy Chem. 2021, 61, 574.
doi: 10.1016/j.jechem.2021.02.012 |
| [26] |
Wu, F.; Dong, J.-Y.; Chen, L.; Bao, L.-Y.; Li, N.; Cao, D.-Y.; Lu, Y.; Xue, R.-X.; Liu, N.; Wei, L.; Wang, Z.-R.; Chen, S.; Su, Y.-F. Energy Storage Mater. 2021, 41, 495.
|
| [27] |
Chen, S.; Wang, Z.-R.; Chen, L.; Liu, N.; Li, N.; Lu, Y.; Cao, D.-Y.; Fu, N.-T.; Li, Q.; Su, Y.-F.; Wu, F. ChemElectroChem 2021 8, 4207.
doi: 10.1002/celc.v8.22 |
| [28] |
Hofmann, M.; Nagler, F.; Kapuschinski, M.; Guntow, U.; Giffin, G.-A. ChemSusChem 2020, 13, 5962.
doi: 10.1002/cssc.v13.22 |
| [29] |
Negi, R.-S.; Culver, S.-P.; Mazilkin, A.; Brezesinski, T.; Elm, M.-T. ACS Appl Mater Interfaces 2020, 12, 31392.
doi: 10.1021/acsami.0c06484 |
| [30] |
Zha, G.-J.; Luo, Y.-P; Hu, N.-G.; Ouyang, C.-Y.; Hou, H.-Q. ACS Appl. Mater. Interfaces 2020, 12, 36046.
doi: 10.1021/acsami.0c07931 |
| [31] |
Li, W.; Li, Y.-J.; Yang, L.-S.; Chen, Y.-X.; Guo, J.; Zhu, J.; Cao, G.-L. Ionics 2020, 26, 5393.
doi: 10.1007/s11581-020-03657-8 |
| [32] |
Li, W.; Yang, L.-S.; Li, Y.-J.; Chen, Y.-X.; Guo, J.; Zhu, J.; Pan, H.; Xi, X.-M. Front. Chem. 2020, 8, 597.
doi: 10.3389/fchem.2020.00597 |
| [33] |
Su, Y.-F.; Zhang, Q.-Y.; Chen, L.; Bao, L.-Y.; Lu, Y.; Chen, S.; Wu, F. Acta Phys.-Chim. Sin. 2021, 37, 110. (in Chinese)
|
|
( 苏岳锋, 张其雨, 陈来, 包丽颖, 卢赟, 陈实, 吴锋, 物理化学学报, 2021, 37, 110.)
|
|
| [34] |
Chang, B.; Kim, J.; Cho, Y.; Hwang, I.; Jung, M.-S.; Char, K.; Lee, K.-T.; Kim, K.-J.; Choi, J.-W. Adv. Energy Mater. 2020, 10, 2001069.
doi: 10.1002/aenm.v10.29 |
| [35] |
Kang, K.-S.; Seong, M.-J.; Oh, S.-H.; Yu, J.-S.; Yim, T. Bull. Korean Chem. Soc. 2020, 41, 1107.
doi: 10.1002/bkcs.v41.11 |
| [36] |
Ma, Y.; Xu, M.; Zhang, J.-B.; Liu, R.; Wang, Y.-M.; Xiao, H.-H.; Huang, Y.-Y.; Yuan, G.-H. J. Alloys Compd 2020, 848, 156387.
doi: 10.1016/j.jallcom.2020.156387 |
| [37] |
Chen, S.; He, T.; Su, Y.-F.; Lu, Y.; Bao, L.-Y.; Chen, L.; Zhang, Q.-Y.; Wang, J.; Chen, R.-J.; Wu, F. ACS Appl. Mater. Interfaces 2017, 9, 29732.
doi: 10.1021/acsami.7b08006 |
| [38] |
Pedaballi, S.; Li, C.-C. J. Power Sources 2020, 472, 228552
doi: 10.1016/j.jpowsour.2020.228552 |
| [39] |
Huang, X.; Zhu, W.-C.; Yao, J.-Y.; Bu, L.-M.; Li, X.-Y.; Tian, K.; Lu, H.; Quan, C.-Q.; Xu, S.-G.; Xu, K.-H.; Jiang, Z.-K.; Zhang, X.; Gao, L.-J.; Zhao, J.-Q. J. Mater. Chem. A 2020, 8, 174291.
|
| [40] |
Zhang, X.-H.; Ma, F.; Wei, G.-Y.; Lei, Z.; Qu, J.-K. J. Solid State Electrochem. 2020, 24, 2301
doi: 10.1007/s10008-020-04742-8 |
| [41] |
Breddemann, U.; Sicklinger, J.; Schipper, F.; Davis, V.; Fischer, A.; Huber, K.; Erickson, E. M.; Daub, M.; Hoffmann, A.; Erk, C.; Markovsky, B.; Aurbach, D.; Gasteiger, H.-A.; Krossing, I. Batteries Supercaps 2020, 4, 632.
doi: 10.1002/batt.v4.4 |
| [42] |
Liu, K.; Zhang, Q.-Q.; Dai, S.; Li, W.; Liu, X.-J.; Ding, F.; Zhang, J.-L. ACS Appl. Mater. Interfaces 2018, 10, 34153.
doi: 10.1021/acsami.8b10016 |
| [43] |
Dong, H.-D.; Tang, C.-Q.; Xu, G.; Liang, H.-Y.; Gao, W.-J. Toothpaste Industry 2015, 25, 30. (in Chinese)
|
|
( 董海德, 唐传勤, 徐钢, 梁红艳, 高万杰, 口腔护理用品工业, 2015, 25, 30.)
|
|
| [44] |
Zuo, J.-D.; Zhao, Y.; Dong, B.-Q.; Chen, Y.-L. Proceedings of 2014 National Symposium on Polymer Materials Science and Engineering, 2014, pp. 374-377. (in Chinese)
|
|
( 左建东, 赵源, 董必钦, 陈义亮, 2014年全国高分子材料科学与工程研讨会学术论文集, 2014, pp. 374-377.)
|
|
| [45] |
Li, X.; Xie, Z.-W.; Liu, W.-J.; Ge, W.-J.; Wang, H.; Qu, M.-Z. Electrochim. Acta 2015, 174, 1122.
doi: 10.1016/j.electacta.2015.06.099 |
| [46] |
Xue, W.; Huang, M.; Li, Y.; Zhu, Y. G.; Gao, R.; Xiao, X.; Zhang, W.; Li, S.; Xu, G.; Yu, Y.; Li, P.; Lopez, J.; Yu, D.; Dong, Y.; Fan, W.; Shi, Z.; Xiong, R.; Sun, C.-J.; Hwang, I.; Lee, W.-K.; Shao-Horn, Y.; Johnson, J. A.; Li, J. Nature Energy 2021, 6, 495.
doi: 10.1038/s41560-021-00792-y |
| [47] |
Yang, W.; Xiang, W.; Chen, Y.-X.; Wu, Z.-G.; Hua, W.-B.; Qiu, L.; He, F.-R.; Zhang, J.; Zhong, B.-H.; Guo, X.-D. ACS Appl. Mater. Interfaces 2020, 12, 10240.
doi: 10.1021/acsami.9b18542 |
| [48] |
Hu, D.-Z.; Su, Y.-F.; Chen, L.; Li, N.; Bao, L.-Y.; Lu, Y.; Zhang, Q.-Y.; Wang, J.; Chen, S.; Wu, F. J. Energy Chem. 2021, 58, 1.
doi: 10.1016/j.jechem.2020.09.031 |
| [49] |
Xiong, X.-H.; Ding, D.; Bu, Y.-F.; Wang, Z.-X.; Huang, B.; Guo, H.-J.; Li, X.-H. J. Mater. Chem. A 2014, 2, 11691.
doi: 10.1039/C4TA01282H |
| [50] |
Wu, F.; Liu, N.; Chen, L.; Su, Y.-F.; Tan, G.-Q.; Bao, L.-Y.; Zhang, Q.-Y.; Lu, Y.; Wang, J.; Chen, S.; Tan, J. Nano Energy 2019, 59, 50.
doi: 10.1016/j.nanoen.2019.02.027 |
| [51] |
Wu, F.; Liu, N.; Chen, L.; Li, N.; Dong, J.-Y.; Lu, Y.; Tan, G.-Q.; Xu, M.-Z.; Cao, D.-Y.; Liu, Y.-F.; Chen, Y.-B.; Su, Y.-F. J. Energy Chem. 2021, 62, 351.
doi: 10.1016/j.jechem.2021.03.035 |
| [52] |
Wu, F.; Liu, N.; Chen, L.; Li, N.; Lu, Y.; Cao, D.-Y.; Xu, M.-Z.; Wang, Z.-R.; Su, Y.-F. ACS Appl. Mater. Interfaces 2021, 13, 24925.
doi: 10.1021/acsami.1c05486 |
| [53] |
Wu, F.; Dong, J.-Y.; Chen, L.; Bao, L.-Y.; Li, N.; Cao, D.-Y.; Lu, Y.; Xue, R.-X.; Liu, N.; Wei, L.; Wang, Z.-R.; Chen, S.; Su, Y.-F. Energy Storage Mater. 2021, 62, 351.
|
| [1] | Shouxiao Chen, Junke Liu, Weichen Zheng, Guozhen Wei, Yao Zhou, Juntao Li. Electron/ion Conductor Double-coated LiNi0.8Co0.1Mn0.1O2 Li-ion Battery Cathode Material and Its Electrochemical Performance [J]. Acta Chimica Sinica, 2022, 80(4): 485-493. |
| [2] | Min Dai, Gangtie Lei, Zhao Zhang, Zhi Li, Hujun Cao, Ping Chen. Room Temperature Hydrogen Absorption of V2O5 Catalyzed MgH2/Mg※ [J]. Acta Chimica Sinica, 2022, 80(3): 303-309. |
| [3] | Tongxin Li, Donglin Li, Qingbo Zhang, Jianhang Gao, Xiangze Kong, Xiaoyong Fan, Lei Gou. Preparation and Electrochemical Performance of Macroporous Ni-rich LiNi0.8Co0.1Mn0.1O2 Cathode Material [J]. Acta Chimica Sinica, 2021, 79(5): 678-684. |
| [4] | Wang Xiaoyu, Zhang Yu, Ma Lei, Wei Liangming. Recent Development on Binders for Silicon-Based Anodes in Lithium-Ion Batteries [J]. Acta Chim. Sinica, 2019, 77(1): 24-40. |
| [5] | Wang Xiao, Li Youbin, Du Lingyu, Gao Fujie, Wu Qiang, Yang Lijun, Chen Qiang, Wang Xizhang, Hu Zheng. Free-Standing Monolithic Sulfur Cathode of Reduced Graphene Oxide Wrapped Sulfur-Filled Carbon Nanocages with High Areal Capacity [J]. Acta Chim. Sinica, 2018, 76(8): 627-632. |
| [6] | Zheng Zhuo, Wu Zhenguo, Xiang Wei, Guo Xiaodong. Preparation and Electrochemical Performance of High Rate Spherical Layered LiNi0.5Co0.2Mn0.3O2 Cathode Material for Lithium-Ion Batteries [J]. Acta Chim. Sinica, 2017, 75(5): 501-507. |
| [7] | Yang Chun, Gong Zhengliang, Zhao Wengao, Yang Yong. Synthesis and Electrochemical Performance of Lithium Rich Cathode Materials xLi3NbO4·(1-x)LiMO2 (M=Mn, Co; 0 < x < 1) for Li-ion Batteries [J]. Acta Chim. Sinica, 2017, 75(2): 212-217. |
| [8] | Tong Zhenkun, Fang Shan, Zheng Hao, Zhang Xiaogang. Zn2GeO4 Nanorods@Graphene Composite as Anode Materials for Li-ion Batteries [J]. Acta Chim. Sinica, 2016, 74(2): 185-190. |
| [9] | Ye Ya, Zhu Jingyi, Yao Yinan, Wang Yuguo, Wu Ping, Tang Yawen, Zhou Yiming, Lu Tianhong. One-pot Synthesis of Sn/Mesoporous Carbon Composite in a Polyol System with Well-improved Lithium Storage Capability [J]. Acta Chim. Sinica, 2015, 73(2): 151-155. |
| [10] | Huang Guoyong, Xu Shengming, Wang Junlian, Li Linyan, Wang Xuejun. Recent Development of Co3O4 and Its Composites as Anode Materials of Lithium-ion Batteries [J]. Acta Chimica Sinica, 2013, 71(12): 1589-1597. |
| [11] | Lu Xia, Wu Renxiang, Li Bobo, Zhu Yunfeng, Li Liquan. Novel PVA/SiO2 Alkaline Micro-porous Polymer Electrolytes for Polymer Ni—MH Batteries [J]. Acta Chimica Sinica, 2013, 71(03): 427-432. |
| [12] | ZHUANG Quan-Chao Shi-Gang Sun. Effects of of Temperature on the Process of Lithium Intercalation-deintercalation in LiCoO2 [J]. Acta Chimica Sinica, 2008, 66(7): 722-728. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||