Acta Chimica Sinica ›› 2022, Vol. 80 ›› Issue (3): 386-394.DOI: 10.6023/A21110536 Previous Articles Next Articles
Review
投稿日期:2021-11-27
发布日期:2021-12-16
通讯作者:
曾庆乐
作者简介: |
王一丁, 成都理工大学2019 级硕士生. 本科毕业于成都理工大学, 硕士阶段师从曾庆乐教授, 主要从事钯催化环化反应和不对称催化反应. |
 |
李福海, 成都理工大学2018级硕士生, 本科毕业于四川理工学院(今更名为四川轻化工大学), 硕士阶段师从曾庆乐教授, 主要从事有机合成方法学和有机硒化学的研究. |
 |
曾庆乐, 教授, 博士生导师, 四川省有突出贡献的优秀专家, 1970年出生于福建省漳州市平和县, 1994年在福建师范大学获得理学学士学位, 1997年在中国科学院兰州化学物理研究所获得理学硕士学位, 2002年在中国科学院成都有机化学研究所获得理学博士学位. 2006年5月入职成都理工大学. 2008年至2009年在美国麻省理工学院做访问科学家. 主要研究方向包括有机合成方法学、(手性)有机硫化学、矿产资源化学和环境治理材料. |
基金资助:
Yiding Wang, Fuhai Li, Qingle Zeng( )
)
Received:2021-11-27
Published:2021-12-16
Contact:
Qingle Zeng
About author:Supported by:Share
Yiding Wang, Fuhai Li, Qingle Zeng. Advances in Formation of C—X Bonds via Cleavage of C—N Bond of Quaternary Ammonium Salts[J]. Acta Chimica Sinica, 2022, 80(3): 386-394.
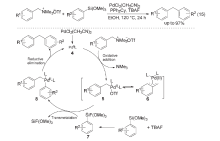
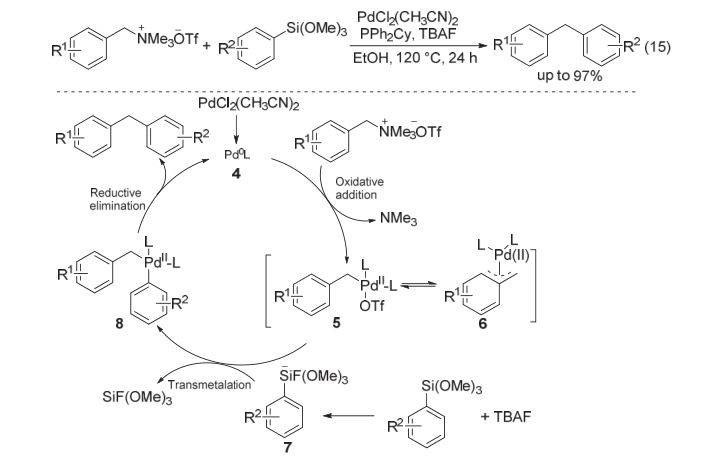
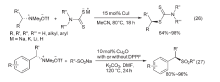
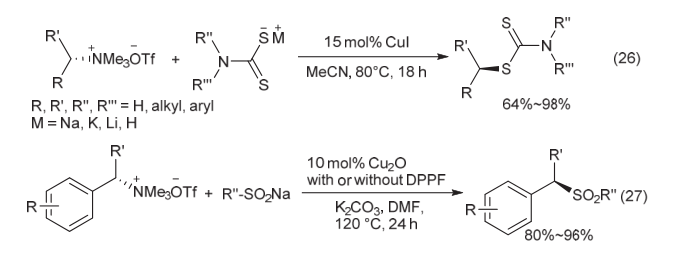
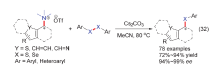

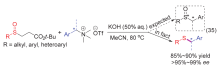
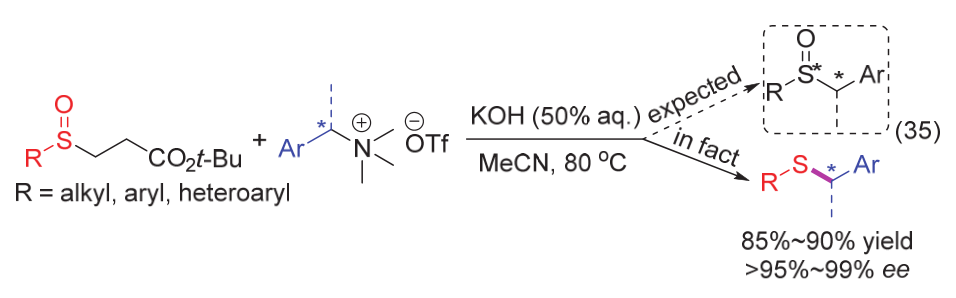
| [1] |
Hartwig, J. F. Acc. Chem. Res. 1998, 31, 852.
doi: 10.1021/ar970282g |
| [2] |
Ley, S. V.; Thomas, A. W. Angew. Chem. Int. Ed. 2003, 42, 5400.
doi: 10.1002/(ISSN)1521-3773 |
| [3] |
Schlummer, B.; Scholz, U. Adv. Synth. Catal. 2004, 346, 1599.
doi: 10.1002/adsc.200404216 |
| [4] |
Ouyang, K.; Hao, W.; Zhang, W. X.; Xi, Z. Chem. Rev. 2015, 115, 12045.
doi: 10.1021/acs.chemrev.5b00386 |
| [5] |
Wang, Q.; Su, Y.; Li, L.; Huang, H. Chem. Soc. Rev. 2016, 45, 1257.
doi: 10.1039/C5CS00534E |
| [6] |
Wang, Z. X.; Yang, B. Org. Biomol. Chem. 2020, 18, 1057.
doi: 10.1039/C9OB02667C |
| [7] |
Li, G.; Chen, Y.; Xia, J. B. Chin. J. Org. Chem. 2018, 38, 1949. (in Chinese)
doi: 10.6023/cjoc201803013 |
|
(李刚, 陈烨, 夏纪宝, 有机化学, 2018, 38, 1949.)
doi: 10.6023/cjoc201803013 |
|
| [8] |
Song, M. M.; Zhang, Z. G.; Zheng, D.; Li, X.; Liang, R.; Zhao, X. N.; Shi, L.; Zhang, G. S. Chin. J. Org. Chem. 2020, 40, 2433. (in Chinese)
doi: 10.6023/cjoc202001007 |
|
(宋蒙蒙, 张志国, 郑丹, 李祥, 梁蕊, 赵旭娜, 时蕾, 张贵生, 有机化学, 2020, 40, 2433.)
doi: 10.6023/cjoc202001007 |
|
| [9] |
Menggen, Q.; Wu, Y.; Bao, Y. S. Chin. J. Org. Chem. 2018, 38, 902. (in Chinese)
|
|
(孟根其其格, 乌云, 包永胜, 有机化学, 2018, 38, 902.)
doi: 10.6023/cjoc201710034 |
|
| [10] |
Zhao, Y.; Li, S. H.; Zhang, M. M.; Liu, F. Acta Chim. Sinica 2019, 77, 916. (in Chinese)
doi: 10.6023/A19040121 |
|
(赵勇, 李施宏, 张苗苗, 刘峰, 化学学报, 2019, 77, 916.)
doi: 10.6023/A19040121 |
|
| [11] |
Liu, J.; Yang, Y.; Ouyang, K.; Zhang, W. X. Green Synth. Catal. 2021, 2, 87.
|
| [12] |
Wang, C. Chem. Pharm. Bull. 2020, 68, 683.
doi: 10.1248/cpb.c20-00196 |
| [13] |
Bao, H.; Qi, X.; Tambar, U. K. J. Am. Chem. Soc. 2011, 133, 1206.
doi: 10.1021/ja110500m |
| [14] |
Wenkert, E.; Han, A. L.; Jenny, C. J. J. Chem. Soc. Chem. Commnu. 1988, 975.
|
| [15] |
Reeves, J. T.; Fandrick, D. R.; Tan, Z.; Song, J. J.; Lee, H.; Yee, N. K.; Senanayake, C. H. Org. Lett. 2010, 12, 4388.
doi: 10.1021/ol1018739 |
| [16] |
Guo, W. J.; Wang, Z. X. Tetrahedron 2013, 69, 9580.
doi: 10.1016/j.tet.2013.09.039 |
| [17] |
Xie, L. G.; Wang, Z. X. Angew. Chem. Int. Ed. 2011, 50, 4901.
doi: 10.1002/anie.v50.21 |
| [18] |
Ogawa, H.; Yang, Z. K.; Minami, H.; Kojima, K.; Saito, T.; Wang, C.; Uchiyama, M. ACS Catal. 2017, 7, 3988.
doi: 10.1021/acscatal.7b01058 |
| [19] |
Wang, D. Y.; Kawahata, M.; Yang, Z. K.; Miyamoto, K.; Komagawa, S.; Yamaguchi, K.; Wang, C.; Uchiyama, M. Nature Commun. 2016, 7, 12937.
doi: 10.1038/ncomms12937 |
| [20] |
Yang, Z. K.; Wang, D. Y.; Minami, H.; Ogawa, H.; Ozaki, T.; Saito, T.; Miyamoto, K.; Wang, C.; Uchiyama, M. Chem. Eur. J. 2016, 22, 15693.
doi: 10.1002/chem.201603436 |
| [21] |
Blakey, S. B.; MacMillan, D. W. C. J. Am. Chem. Soc. 2003, 125, 6046.
pmid: 12785821 |
| [22] |
Maity, P.; Shacklady-McAtee, D. M.; Yap, G. P. A.; Sirianni, E. R.; Watson, M. P. J. Am. Chem. Soc. 2013, 135, 280.
doi: 10.1021/ja3089422 |
| [23] |
Chen, Q.; Gao, F.; Tang, H.; Yao, M.; Zhao, Q.; Shi, Y.; Dang, Y.; Cao, C. ACS Catal. 2019, 9, 3730.
doi: 10.1021/acscatal.9b00218 |
| [24] |
Xu, S.; Zhang, Z.; Han, C.; Hu, W.; Xiao, T.; Yuan, Y.; Zhao, J. J. Org. Chem. 2019, 84, 12192.
doi: 10.1021/acs.joc.9b01877 |
| [25] |
Zhu, F.; Tao, J. L.; Wang, Z. X. Org. Lett. 2015, 17, 4926.
doi: 10.1021/acs.orglett.5b02458 |
| [26] |
Han, C.; Zhang, Z.; Xu, S.; Wang, K.; Chen, K.; Zhao, J. J. Org. Chem. 2019, 84, 16308.
doi: 10.1021/acs.joc.9b02554 |
| [27] |
Moragas, T.; Gaydou, M.; Martin, R. Angew. Chem. Int. Ed. 2016, 55, 5053.
doi: 10.1002/anie.v55.16 |
| [28] |
Liao, L. L.; Cao, G. M.; Ye, J. H.; Sun, G. Q.; Zhou, W. J.; Gui, Y. Y.; Yan, S. S.; Shen, G.; Yu, D. G. J. Am. Chem. Soc. 2018, 140, 17338.
doi: 10.1021/jacs.8b08792 pmid: 30518213 |
| [29] |
Yu, W.; Yang, S.; Xiong, F.; Fan, T.; Feng, Y.; Huang, Y.; Fu, J.; Wang, T. Org. Biomol. Chem. 2018, 16, 3099.
doi: 10.1039/C8OB00488A |
| [30] |
Rand Alexander, W.; Montgomery, J. Chem. Sci. 2019, 10, 5338.
doi: 10.1039/c9sc01083a pmid: 31191891 |
| [31] |
Scharfbier, J.; Gross, B. M.; Oestreich, M. Angew. Chem. Int. Ed. 2020, 59, 1577.
doi: 10.1002/anie.v59.4 |
| [32] |
Zhang, X. Q.; Wang, Z. X. Org. Biomol. Chem. 2014, 12, 1448.
doi: 10.1039/c3ob41989d |
| [33] |
Chen, H.; Yang, H.; Li, N.; Xue, X.; He, Z.; Zeng, Q. Org. Proc. Res. Dev. 2019, 23, 1679.
|
| [34] |
Yang, B.; Wang, Z. X. J. Org. Chem. 2019, 84, 1500.
doi: 10.1021/acs.joc.8b02926 pmid: 30628791 |
| [35] |
Li, N.; Chen, F.; Wang, G.; Zeng, Q. Monatsch. Chem. 2020, 151, 99.
doi: 10.1007/s00706-019-02535-y |
| [36] |
O'Connor, S. E.; Grosset, A.; Janiak, P. Fund. Clin. Pharmacol. 1999, 13, 145.
pmid: 10226758 |
| [37] |
Sobal, G.; Menzel, E. J.; Sinzinger, H. Biochem. Pharmacol. 2001, 61, 373.
pmid: 11172743 |
| [38] |
Zeng, Q.; Wang, H.; Wang, T.; Cai, Y.; Weng, W.; Zhao, Y. Adv. Synth. Catal. 2005, 347, 1933.
doi: 10.1002/(ISSN)1615-4169 |
| [39] |
Zhang, L.; Tan, M.; Zhou, L.; Zeng, Q. Tetrahedron Lett. 2018, 59, 2778.
doi: 10.1016/j.tetlet.2018.06.008 |
| [40] |
Jiang, W.; Huang, Y.; Zhou, L.; Zeng, Q. Sci. China Chem. 2019, 62, 1213.
doi: 10.1007/s11426-019-9499-5 |
| [41] |
Feng, J.; Zhang, Q.; Li, F.; Yang, L.; Kuchukulla, R. R.; Zeng, Q. Synlett 2021, 32, 224.
doi: 10.1055/s-0040-1707319 |
| [42] |
Jiang, W.; Li, N.; Zhou, L.; Zeng, Q. ACS Catal. 2018, 8, 9899.
doi: 10.1021/acscatal.8b03032 |
| [43] |
Chen, H.; Jiang, W.; Zeng, Q. Chem. Rec. 2020, 20, 1269.
doi: 10.1002/tcr.v20.11 |
| [44] |
Chen, H.; Zhang, Q.; Zheng, W.; Yang, H.; Zeng, Q. Asian J. Org. Chem. 2020, 9, 773.
doi: 10.1002/ajoc.v9.5 |
| [45] |
Huang, Y.; Chen, H.; Zheng, W.; Zeng, Q. Tetrahedron Lett. 2020, 61, 152320.
doi: 10.1016/j.tetlet.2020.152320 |
| [46] |
Huang, Y.; Li, J.; Chen, H.; He, Z.; Zeng, Q. Chem. Rec. 2021, 21, 1216.
doi: 10.1002/tcr.v21.5 |
| [47] |
Zhang, H.; Hagihara, S.; Itami, K. Chem. Eur. J. 2015, 21, 16796.
doi: 10.1002/chem.v21.47 |
| [48] |
Hu, J.; Sun, H.; Cai, W.; Pu, X.; Zhang, Y.; Shi, Z. J. Org. Chem. 2016, 81, 14.
doi: 10.1021/acs.joc.5b02557 |
| [49] |
Basch, C. H.; Cobb, K. M.; Watson, M. P. Org. Lett. 2016, 18, 136.
doi: 10.1021/acs.orglett.5b03455 |
| [50] |
Gui, Y.; Tian, S. K. Org. Lett. 2017, 19, 1554.
doi: 10.1021/acs.orglett.7b00365 |
| [51] |
Li, F.; Wang, D.; Chen, H.; He, Z.; Zhou, L.; Zeng, Q. Chem. Commun. 2020, 56, 13029.
doi: 10.1039/D0CC05633B |
| [52] |
Tang, Q.; Li, F.; Chen, F.; Yin, X.; Tang, Y.; Zeng, Q. Asian J. Org. Chem. 2021, 10, 1687.
doi: 10.1002/ajoc.v10.7 |
| [53] |
Chen, F.; Li, F.; Zeng, Q. Eur. J. Org. Chem. 2021, 2021, 5605.
doi: 10.1002/ejoc.v2021.41 |
| [54] |
Zhang, Q.; Feng, H.; Yang, H.; He, Z.; Zeng, Q. J. Org. Chem. 2021, 86, 7806.
doi: 10.1021/acs.joc.1c00615 |
| [55] |
Yang, L.; Wang, B.; Yin, X.; Zeng, Q. Chem. Rec. 2021, DOI: 10.1002/tcr.202100242.
doi: 10.1002/tcr.202100242 |
| [56] |
Mfuh, A. M.; Doyle, J. D.; Chhetri, B.; Arman, H. D.; Larionov, O. V. J. Am. Chem. Soc. 2016, 138, 2985.
doi: 10.1021/jacs.6b01376 |
| [57] |
Wang, D. Y.; Yang, Z. K.; Wang, C.; Zhang, A.; Uchiyama, M. Angew. Chem. Int. Ed. 2018, 57, 3641.
doi: 10.1002/anie.201712618 |
| [58] |
Wang, D. Y.; Wen, X.; Xiong, C. D.; Zhao, J. N.; Ding, C. Y.; Meng, Q.; Zhou, H.; Wang, C.; Uchiyama, M.; Lu, X. J.; Zhang, A. iScience 2019, 15, 307.
doi: 10.1016/j.isci.2019.04.038 |
| [59] |
Yang, D. T.; Zhu, M.; Schiffer, Z. J.; Williams, K.; Song, X.; Liu, X.; Manthiram, K. ACS Catal. 2019, 9, 4699.
doi: 10.1021/acscatal.9b00818 |
| [1] | Liu Ruxue, He Xiaoyan, Niu Litong, Lv Bolin, Yu Fei, Zhang Zhe, Yang Zhiwang. Hierarchical In2S3/CdIn2S4 Heterostructured Nanohybrids as Photocatalyst for Coupling of Benzyl Amines under Visible Light [J]. Acta Chim. Sinica, 2019, 77(7): 653-660. |
| [2] | Zhang Zhaoxiang, Liu Yujie, Wang Qi, Wang Jingjing. Capillary Electrophoresis and Quantum Dot Electrochemiluminescence by Micellar Reversed Sweeping [J]. Acta Chim. Sinica, 2019, 77(2): 179-183. |
| [3] | Li Xuefei, Chen Ling, Xu Shengchao, Zhao Wenbo. Liquid-liquid Phase-change Absorption of SO2 Using N,N-Dimethyl-n-octylamine Mixed with Hexadecane [J]. Acta Chimica Sinica, 2019, 77(12): 1287-1293. |
| [4] | Zhang Yongling, Wang Min, Cao Peng, Liao Jian. Copper-Catalyzed Enantioselective Aminoboration of Styrenes with Chiral Sulfoxide Phosphine Ligand [J]. Acta Chim. Sinica, 2017, 75(8): 794-797. |
| [5] | Zhang Binbin, Zhan Dan, Zhang Xiaoping, Xiang Qinjie, Zeng Qingle. Ligand-free Pd-Catalyzed C—N Coupling of Diphenylamine and Aryl Halides under Air [J]. Acta Chimica Sinica, 2012, 70(15): 1655-1659. |
| [6] | ZHOU Xin, SHANG Jia, LIU Ming-Ming, LIU Han-Lan, HAO Rong, FENG Xiong-Han, LIU Fan. Preparation of Novel Ionic Liquids-Based Sol-Gel Coatings for Solid-Phase Microextraction [J]. Acta Chimica Sinica, 2010, 68(17): 1749-1757. |
| [7] | . Synthesis and Spectrum Stability of an Oligomeric Terfluorene Tethered with Antioxidant Hindered Amine [J]. Acta Chimica Sinica, 2008, 26(23): 2575-2578. |
| [8] | WANG Gui-Xiang; XIAO He-Ming*; JU Xue-Hai; GONG Xue-Dong. Theoretical Studies on Densities, Detonation Velocities and Pres-sures and Electric Spark Sensitivities of Energetic Materials [J]. Acta Chimica Sinica, 2007, 65(6): 517-524. |
| [9] | Liu Hongmin;Zhang Fuyi;Xu Wen;Li Shi. Synthesis of Aminosugars Having New Structures from Oxosugars [J]. Acta Chimica Sinica, 2003, 61(7): 1149-1152. |
| [10] | Yuan Qiaolong;Wang Dening;Wu Shusen;Ying Shengkang. Adsorption of Poly(urethane-urea-amine) Acetate on Colloidal Silica [J]. Acta Chimica Sinica, 2003, 61(10): 1543-1549. |
| [11] | Gao Xiang;Zhang Xiaoyue;Zhang Danwei;Liu Ying;Wu Shihui. A Thermoinduced [3+2] Cycloaddition of Allylic Amines with C_(60) [J]. Acta Chimica Sinica, 2003, 61(10): 1686-1691. |
| [12] | Sun Xiaoling;Li Xingya;Jiang Xikui;Fu Wimin. Novel Reactions of Enamines with Perhaloethanes: A Facile Route to β-CF_3 Substituted α, β-Unsaturated Ketones [J]. Acta Chimica Sinica, 2003, 61(10): 1641-1645. |
| [13] | Song Maoying;Wang Xiqing;Qian Bin;Zeng Yu;Long Yingcai. Studies on Properties of Pure-silica β Zeolite [J]. Acta Chimica Sinica, 2002, 60(3): 451-456. |
| [14] | Guo Gangjun;Ma lin;Mao Xuepu;Gu Lianquan;Wang Jun. A Novel Arylamine-o-quinone Polymer: Synthesis and Application as a Nano-support for Immobilized Enzymes [J]. Acta Chimica Sinica, 2002, 60(3): 499-503. |
| [15] | Shi Feng;Deng Youquan. Synthesis of alkylformamide catalyzed by organic Au(Ⅰ) complexes [J]. Acta Chimica Sinica, 2001, 59(6): 979-981. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||