Acta Chimica Sinica ›› 2022, Vol. 80 ›› Issue (1): 16-21.DOI: 10.6023/A21100467 Previous Articles Next Articles
Special Issue: 中国科学院青年创新促进会合辑
Article
李玲玲a,b, 刘宇a,b, 宋术岩a,b,*( ), 张洪杰a,b,c,*(
), 张洪杰a,b,c,*( )
)
投稿日期:2021-10-20
发布日期:2021-12-06
通讯作者:
宋术岩, 张洪杰
作者简介:基金资助:
Lingling Lia,b, Yu Liua,b, Shuyan Songa,b( ), Hongjie Zhanga,b,c(
), Hongjie Zhanga,b,c( )
)
Received:2021-10-20
Published:2021-12-06
Contact:
Shuyan Song, Hongjie Zhang
About author:Supported by:Share
Lingling Li, Yu Liu, Shuyan Song, Hongjie Zhang. Synthesis of Cu Single Atom with Adjustable Coordination Environment and Its Catalytic Hydrogenation Performance※[J]. Acta Chimica Sinica, 2022, 80(1): 16-21.


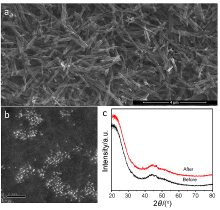

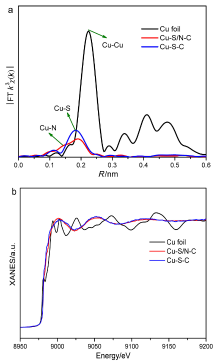


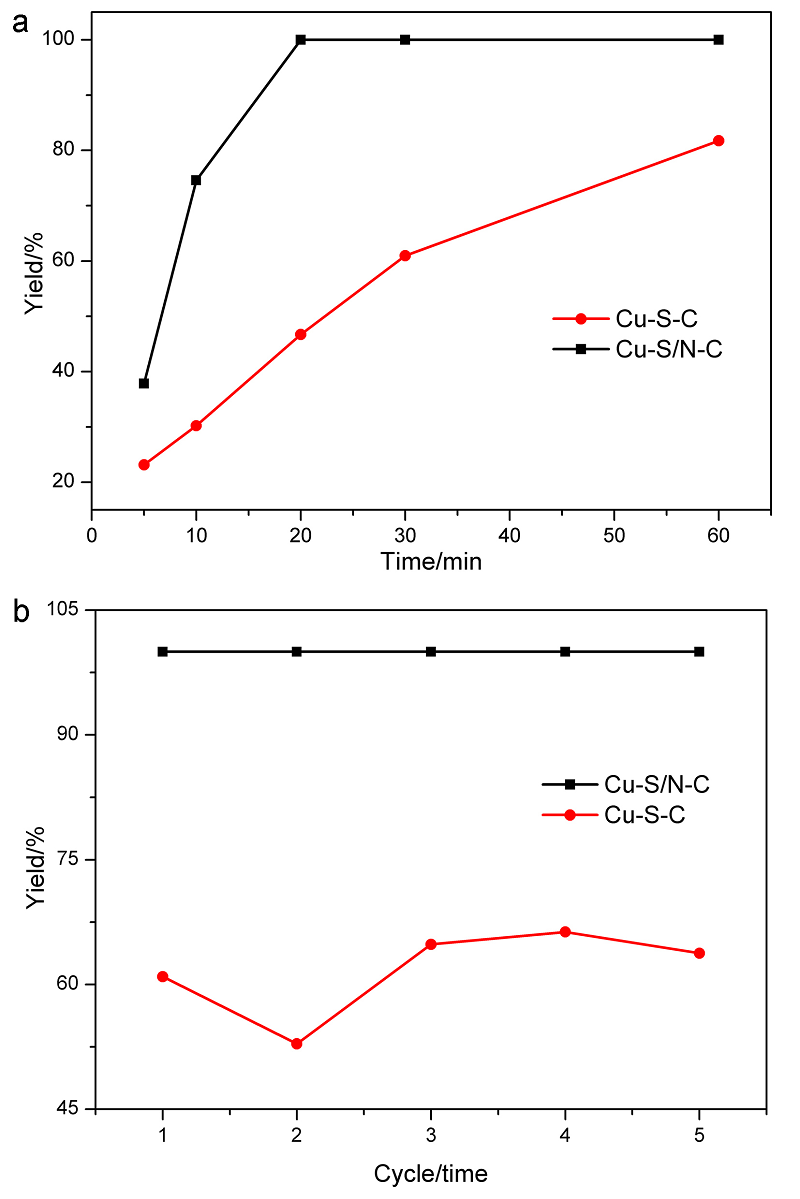
| [1] |
Shuai, X. M.; Shen, W. Z. Nanoscale Res. Lett. 2012, 7, 1.
doi: 10.1186/1556-276X-7-1 |
| [2] |
Kundu, S.; Kundu, P.; Van Tendeloo, G.; Ravishankar, N. Small 2014, 10, 3895.
doi: 10.1002/smll.201400524 |
| [3] |
Zhang, H. B.; Pan, X. L.; Han, X. W.; Liu, X. M.; Wang, X. F.; Shen, W. L.; Bao, X. H. Chem. Sci. 2013, 4, 1075.
doi: 10.1039/c2sc21761a |
| [4] |
Wang, J.; Lei, M.; Wang, Z.; Liu, Y.; Zhuang, W.; Zhu, W. Appl. Surf. Sci. 2021, 542, 148541.
doi: 10.1016/j.apsusc.2020.148541 |
| [5] |
Cao, Y. H.; Guo, L.; Dan, M.; Doronkin, D. E.; Han, C. Q.; Rao, Z. Q.; Liu, Y.; Meng, J.; Huang, Z.; Zheng, K. B.; Chen, P.; Dong, F.; Zhou, Y. Nat. Commun. 2021, 12, 1675.
doi: 10.1038/s41467-021-21925-7 |
| [6] |
Wang, X.; Liu, D.; Song, S.; Zhang, H. J. Am. Chem. Soc. 2013, 135, 15864.
doi: 10.1021/ja4069134 |
| [7] |
Baskaran, S.; Xu, C.-Q.; Wang, Y.-G.; Garzón, I. L.; Li, J. Sci. China Mater. 2020, 63, 993.
doi: 10.1007/s40843-019-1257-x |
| [8] |
Li, F.; Li, Y.; Zeng, X. C.; Chen, Z. ACS Catal. 2014, 5, 544.
doi: 10.1021/cs501790v |
| [9] |
Wang, Q. S.; Zhang, Z. S.; Wang, H.; Liu, Y.; Song, S. Y.; Zhang, H. J. Chem. J. Chin. Univ. 2020, 41, 947. (in Chinese)
|
|
( 王启舜, 张泽树, 王欢, 刘宇, 宋术岩, 张洪杰, 高等学校化学学报, 2020, 41, 947.)
|
|
| [10] |
Wang, F.; Song, S. Y.; Li, K.; Li, J. Q.; Pan, J.; Yao, S.; Ge, X.; Feng, J.; Wang, X.; Zhang, H. J. Adv. Mater. 2016, 28, 10679.
doi: 10.1002/adma.201603608 |
| [11] |
Zhou, H.; Zhao, Y.; Gan, J.; Xu, J.; Wang, Y.; Lv, H.; Fang, S.; Wang, Z.; Deng, Z.; Wang, X.; Liu, P.; Guo, W.; Mao, B.; Wang, H.; Yao, T.; Hong, X.; Wei, S.; Duan, X.; Luo, J.; Wu, Y. J. Am. Chem. Soc. 2020, 142, 12643.
doi: 10.1021/jacs.0c03415 |
| [12] |
Deng, Z. J.; Ouyang, Y. F.; Ao, Y. L.; Cai, Q. Acta Chim. Sinica 2021, 79, 649. (in Chinese)
doi: 10.6023/A21010006 |
|
( 邓卓基, 欧阳溢凡, 敖运林, 蔡倩, 化学学报 2021, 79, 649.)
|
|
| [13] |
Fei, H. L.; Dong, J. C.; Arellano-Jimenez, M. J.; Ye, G. L.; Kim, N. D.; Samuel, E. L. G.; Peng, Z. W.; Zhu, Z.; Qin, F.; Bao, J. M.; Yacaman, M. J.; Ajayan, P. M.; Chen, D. L.; Tour, J. M. Nat. Commun. 2015, 6, 8.
|
| [14] |
Wan, J. W.; Chen, W. X.; Jia, C. Y.; Zheng, L. R.; Dong, J. C.; Zheng, X. S.; Wang, Y.; Yan, W. S.; Chen, C.; Peng, Q.; Wang, D. S.; Li, Y. D. Adv. Mater. 2018, 30, 8.
|
| [15] |
Wan, X.; Liu, X. F.; Li, Y. C.; Yu, R. H.; Zheng, L. R.; Yan, W. S.; Wang, H.; Xu, M.; Shui, J. L. Nat. Catal. 2019, 2, 259.
doi: 10.1038/s41929-019-0237-3 |
| [16] |
Jin, H. H.; Zhou, H.; Ji, P. X.; Zhang, C. T.; Luo, J. H.; Zeng, W. H.; Hu, C. X.; He, D. P.; Mu, S. C. Nano Res. 2020, 13, 818.
doi: 10.1007/s12274-020-2702-3 |
| [17] |
Hu, M. Y.; Li, S. N.; Zheng, S. S.; Liang, X. H.; Zheng, J. X.; Pan, F. J. Phys. Chem. C 2020, 124, 13168.
doi: 10.1021/acs.jpcc.0c01998 |
| [18] |
Li, L. L.; Chang, X.; Lin, X. Y.; Zhao, Z. J.; Gong, J. L. Chem. Soc. Rev. 2020, 49, 8156.
doi: 10.1039/D0CS00795A |
| [19] |
Chen, G. B.; Wang, T.; Liu, P.; Liao, Z. Q.; Zhong, H. X.; Wang, G.; Zhang, P. P.; Yu, M. H.; Zschech, E.; Chen, M. W.; Zhang, J.; Feng, X. L. Energ. Environ. Sci. 2020, 13, 2849.
doi: 10.1039/D0EE01613F |
| [20] |
Lu, X. Q.; Cao, S. F.; Wei, X. F.; Li, S. R.; Wei, S. X. Acta Chim. Sinica 2020, 78, 1001. (in Chinese)
doi: 10.6023/A20060223 |
|
( 鲁效庆, 曹守福, 魏晓飞, 李邵仁, 魏淑贤, 化学学报 2020, 78, 1001.)
|
|
| [21] |
Chen, Y. J.; Gao, R.; Ji, S. F.; Li, H. J.; Tang, K.; Jiang, P.; Hu, H. B.; Zhang, Z. D.; Hao, H. G.; Qu, Q. Y.; Liang, X.; Chen, W. X.; Dong, J. C.; Wang, D. S.; Li, Y. D. Angew. Chem. Int. Ed. 2020, 60, 3212.
doi: 10.1002/anie.v60.6 |
| [22] |
Chen, K. J.; Liu, K.; An, P. D.; Li, H. J. W.; Lin, Y. Y.; Hu, J. H.; Jia, C. K.; Fu, J. W.; Li, H. M.; Liu, H.; Lin, Z.; Li, W. Z.; Li, J. H.; Lu, Y. R.; Chan, T. S.; Zhang, N.; Liu, M. Nat. Commun. 2020, 11, 4173.
doi: 10.1038/s41467-020-18062-y |
| [23] |
Zhu, J.; Ren, Z.; Du, S.; Xie, Y.; Wu, J.; Meng, H.; Xue, Y.; Fu, H. Nano Res. 2017, 10, 1819-1831.
doi: 10.1007/s12274-017-1511-9 |
| [24] |
Jia, Y.; Jiang, K.; Wang, H.; Yao, X. Chem. 2019, 5, 1371-1397.
doi: 10.1016/j.chempr.2019.02.008 |
| [25] |
Wang, Y. S.; Zhao, Y. L.; Zhao, Z. Z.; Lan, X. L.; Xu, J. X.; Xu, W. X.; Duan, Z. K. Acta Chim. Sinica 2019, 77, 661. (in Chinese)
doi: 10.6023/A19040124 |
|
( 王永胜, 赵云鹭, 赵珍珍, 兰小林, 徐金霞, 徐伟祥, 段正康, 化学学报 2019, 77, 661.)
|
|
| [26] |
Shang, H. S.; Zhou, X. G.; Dong, J. C.; Li, A.; Zhao, X.; Liu, Q. H.; Lin, Y.; Pei, J. J.; Li, Z.; Jiang, Z. L.; Zhou, D. N.; Zheng, L. R.; Wang, Y.; Zhou, J.; Yang, Z. K.; Cao, R.; Sarangi, R.; Sun, T. T.; Yang, X.; Zheng, X. S.; Yan, W. S.; Zhuang, Z. B.; Li, J.; Chen, W. X.; Wang, D. S.; Zhang, J. T.; Li, Y. D. Nat. Commun. 2020, 11, 3049.
doi: 10.1038/s41467-020-16848-8 |
| [27] |
Li, Y.; Cheng, W.; Su, H.; Zhao, X.; He, J.; Liu, Q. Nano Energy 2020, 77, 105121.
doi: 10.1016/j.nanoen.2020.105121 |
| [28] |
Zhang, J.; Zhao, Y.; Chen, C.; Huang, Y.-C.; Dong, C.-L.; Chen, C.-J.; Liu, R.-S.; Wang, C.; Yan, K.; Li, Y.; Wang, G. J. Am. Chem. Soc. 2019, 141, 20118.
doi: 10.1021/jacs.9b09352 |
| [29] |
Che, M. Catal. Today 2013, 218, 162.
|
| [30] |
Ramaswamy, N.; Tylus, U.; Jia, Q.; Mukerjee, S. J. Am. Chem. Soc. 2013, 135, 15443.
doi: 10.1021/ja405149m |
| [31] |
Shang, H.; Sun, W.; Sui, R.; Pei, J.; Zheng, L.; Dong, J.; Jiang, Z.; Zhou, D.; Zhuang, Z.; Chen, W.; Zhang, J.; Wang, D.; Li, Y. Nano Lett. 2020, 20, 5443.
doi: 10.1021/acs.nanolett.0c01925 |
| [32] |
Li, X.; Bi, W.; Chen, M.; Sun, Y.; Ju, H.; Yan, W.; Zhu, J.; Wu, X.; Chu, W.; Wu, C.; Xie, Y. J. Am. Chem. Soc. 2017, 139, 14889.
doi: 10.1021/jacs.7b09074 |
| [33] |
Chen, J. Y.; Li, H.; Fan, C. A.; Meng, Q. W.; Tang, Y. W.; Qiu, X. Y.; Fu, G. T.; Ma, T. Y. Adv. Mater. 2020, 32, 2003134.
doi: 10.1002/adma.v32.30 |
| [34] |
Sun, Y.; Silvioli, L.; Sahraie, N. R.; Ju, W.; Li, J.; Zitolo, A.; Li, S.; Bagger, A.; Arnarson, L.; Wang, X.; Moeller, T.; Bernsmeier, D.; Rossmeisl, J.; Jaouen, F.; Strasser, P. J. Am. Chem. Soc. 2019, 141, 12372.
doi: 10.1021/jacs.9b05576 |
| [1] | Luyao Yu, Zhen Ren, Yusen Yang, Min Wei. Directed Preparation of Biomass-based Polyester Monomers by Catalytic Conversion [J]. Acta Chimica Sinica, 2023, 81(2): 175-190. |
| [2] | Xiaomeng Zhang, Xiya Li, Wanfeng Xiong, Hongfang Li, Rong Cao. Ultrafine Platinum Nanoparticles Derived from Supramolecular Crystal for Catalytic Hydrogenation of Nitroarenes [J]. Acta Chimica Sinica, 2021, 79(2): 180-185. |
| [3] | Liu Jianguo, Zhang Mingyue, Wang Nan, Wang Chenguang, Ma Longlong. Research Progress of Covalent Organic Framework Materials in Catalysis [J]. Acta Chimica Sinica, 2020, 78(4): 311-325. |
| [4] | Guo Xiaoling, Chen Xiao, Su Dangsheng, Liang Changhai. Preparation of Ni/C Core-shell Nanoparticles through MOF Pyrolysis for Phenylacetylene Hydrogenation Reaction [J]. Acta Chim. Sinica, 2018, 76(1): 22-29. |
| [5] | Zhang Wen-Qiang, Li Qiu-Yan, Yang Xinyu, Ma Zheng, Wang Huanhuan, Wang Xiao-Jun. Benzothiadiazole Conjugated Metalorganic Framework for Organic Aerobic Oxidation Reactions under Visible Light [J]. Acta Chim. Sinica, 2017, 75(1): 80-85. |
| [6] | Huang Gang, Chen Yuzhen, Jiang Hailong. Metal-Organic Frameworks for Catalysis [J]. Acta Chimica Sinica, 2016, 74(2): 113-129. |
| [7] | Wang Changan, Wang Wei. Advances in Porous Organic Catalysis [J]. Acta Chim. Sinica, 2015, 73(6): 498-529. |
| [8] | Li Zhaohao, Chen Jing, Su Weiping, Hong Maochun. Pd/MgO:A Recyclable, Ligand-free Heterogeneous Catalyst for Decarboxylative Cross-Coupling of Arene Carboxylic Acids with Aryl Halides [J]. Acta Chimica Sinica, 2014, 72(5): 552-556. |
| [9] | YIN Chuan-Qi,quanwu feng,zhengwu bai. Steric Hindrance Effect on Hydrogenation of Styrene Catalyzed by Ruthenium Hydride Complex [J]. Acta Chimica Sinica, 2009, 67(6): 519-522. |
| [10] | . Novel Highly Efficient Cu/HMS Catalyst and Its Application to the Hydrogenation of Dimethyl Oxalate to Ethylene Glycol [J]. Acta Chimica Sinica, 2009, 67(15): 1731-1736. |
| [11] | Zhao Yongxiang;Wu Zhigang;Xu Linping;Zhang Linqing;Liu Diansheng;Xu Xianlun. Effect of Nickel Precursors on the Catalytic Performance of NiO/SiO_2 [J]. Acta Chimica Sinica, 2002, 60(4): 596-599. |
| [12] | Shen Bairong;Fang Zhigang;Fan Kangnian;Deng Jingfa. Theorectical study on the structure and catalytic activity of Ni-Co- B amorphous alloy [J]. Acta Chimica Sinica, 1999, 57(4): 366-371. |
| [13] | Wang Duolu;Zhang Zepeng;Chen Ligong;Xu Zhengshuang;Wang Yi;Huang Hongmei;MENG. The catalysts for the hydrogenation of 2, 2, 6, 6-tetramethyl-4- piperidone to 2, 2, 6, 6-tetramethyl-4-piperidinol [J]. Acta Chimica Sinica, 1999, 57(2): 176-182. |
| [14] | ZHANG JUNLIANG;CAI MENGSHEN. Studies on nucleosidesⅤ: Total synthesis of diacetyl neosidomycin [J]. Acta Chimica Sinica, 1996, 54(1): 84-89. |
| [15] | CHEN HAIYING;DENG JINGFA;SHENG SHISHAN;CHEN HENGRONG;XIONG GUOXING. Studies of amorphous metals as catalytic material.V. amorphous Ni-W-P alloy powder and its hydrogenation activity [J]. Acta Chimica Sinica, 1994, 52(9): 877-882. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||