Acta Chimica Sinica ›› 2022, Vol. 80 ›› Issue (3): 282-290.DOI: 10.6023/A21120556 Previous Articles Next Articles
Article
张璐璐a, 王媛媛a, 朱贵楠b, 戴文博b, 赵紫璇a, 赵盈a, 支俊格a,*( ), 董宇平b
), 董宇平b
投稿日期:2021-12-12
发布日期:2022-01-22
通讯作者:
支俊格
基金资助:
Lulu Zhanga, Yuanyuan Wanga, Guinan Zhub, Wenbo Daib, Zixuan Zhaoa, Ying Zhaoa, Junge Zhia( ), Yuping Dongb
), Yuping Dongb
Received:2021-12-12
Published:2022-01-22
Contact:
Junge Zhi
Supported by:Share
Lulu Zhang, Yuanyuan Wang, Guinan Zhu, Wenbo Dai, Zixuan Zhao, Ying Zhao, Junge Zhi, Yuping Dong. Aggregation-Induced Emission and Mechanochromism of the Tetraphenylbutadiene Derivatives Containing Different Alkyl Chains[J]. Acta Chimica Sinica, 2022, 80(3): 282-290.
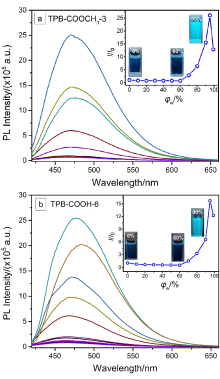

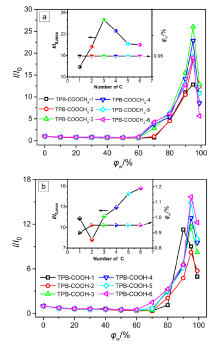
| 样品 | 溶液a | 固体 | αAIEd | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| λabs/nm | λem/nm | Δλb/nm | Δνstb/cm-1 | ΦPLc/% | I/I0 | λem/nm | ΦPLc/% | |||||||
| TPB-COOCH3-1 | 368 | 470 | 102 | 5934 | 3.7 | 12.8 | 462 | 76.5 | 20.5 | |||||
| TPB-COOCH3-2 | 368 | 470 | 102 | 5934 | 3.8 | 18.5 | 460 | 80.1 | 20.6 | |||||
| TPB-COOCH3-3 | 370 | 472 | 102 | 5840 | 1.8 | 26.0 | 472 | 87.3 | 49.6 | |||||
| TPB-COOCH3-4 | 373 | 471 | 98 | 5614 | 1. 9 | 22.9 | 470 | 89.1 | 47.1 | |||||
| TPB-COOCH3-5 | 370 | 471 | 101 | 5796 | 2.1 | 19.3 | 470 | 87.5 | 41.9 | |||||
| TPB-COOCH3-6 | 370 | 471 | 101 | 5796 | 2.2 | 19.0 | 470 | 71.3 | 32.4 | |||||
| TPB-COOH-1 | 365 | 471 | 106 | 6166 | 3.9 | 11.3 | 496 | 55.6 | 14.4 | |||||
| TPB-COOH-2 | 365 | 470 | 105 | 6120 | 2.4 | 8.2 | 480 | 34.5 | 14.5 | |||||
| TPB-COOH-3 | 368 | 471 | 103 | 5979 | 2.1 | 11.6 | 500 | 42.8 | 20.8 | |||||
| TPB-COOH-4 | 368 | 472 | 104 | 6024 | 2.4 | 12.9 | 490 | 52.3 | 22.2 | |||||
| TPB-COOH-5 | 368 | 475 | 107 | 6158 | 2.3 | 14.9 | 492 | 60.9 | 26.3 | |||||
| TPB-COOH-6 | 368 | 475 | 107 | 6158 | 3.0 | 15.7 | 493 | 73.2 | 24.7 | |||||
| 样品 | 溶液a | 固体 | αAIEd | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| λabs/nm | λem/nm | Δλb/nm | Δνstb/cm-1 | ΦPLc/% | I/I0 | λem/nm | ΦPLc/% | |||||||
| TPB-COOCH3-1 | 368 | 470 | 102 | 5934 | 3.7 | 12.8 | 462 | 76.5 | 20.5 | |||||
| TPB-COOCH3-2 | 368 | 470 | 102 | 5934 | 3.8 | 18.5 | 460 | 80.1 | 20.6 | |||||
| TPB-COOCH3-3 | 370 | 472 | 102 | 5840 | 1.8 | 26.0 | 472 | 87.3 | 49.6 | |||||
| TPB-COOCH3-4 | 373 | 471 | 98 | 5614 | 1. 9 | 22.9 | 470 | 89.1 | 47.1 | |||||
| TPB-COOCH3-5 | 370 | 471 | 101 | 5796 | 2.1 | 19.3 | 470 | 87.5 | 41.9 | |||||
| TPB-COOCH3-6 | 370 | 471 | 101 | 5796 | 2.2 | 19.0 | 470 | 71.3 | 32.4 | |||||
| TPB-COOH-1 | 365 | 471 | 106 | 6166 | 3.9 | 11.3 | 496 | 55.6 | 14.4 | |||||
| TPB-COOH-2 | 365 | 470 | 105 | 6120 | 2.4 | 8.2 | 480 | 34.5 | 14.5 | |||||
| TPB-COOH-3 | 368 | 471 | 103 | 5979 | 2.1 | 11.6 | 500 | 42.8 | 20.8 | |||||
| TPB-COOH-4 | 368 | 472 | 104 | 6024 | 2.4 | 12.9 | 490 | 52.3 | 22.2 | |||||
| TPB-COOH-5 | 368 | 475 | 107 | 6158 | 2.3 | 14.9 | 492 | 60.9 | 26.3 | |||||
| TPB-COOH-6 | 368 | 475 | 107 | 6158 | 3.0 | 15.7 | 493 | 73.2 | 24.7 | |||||

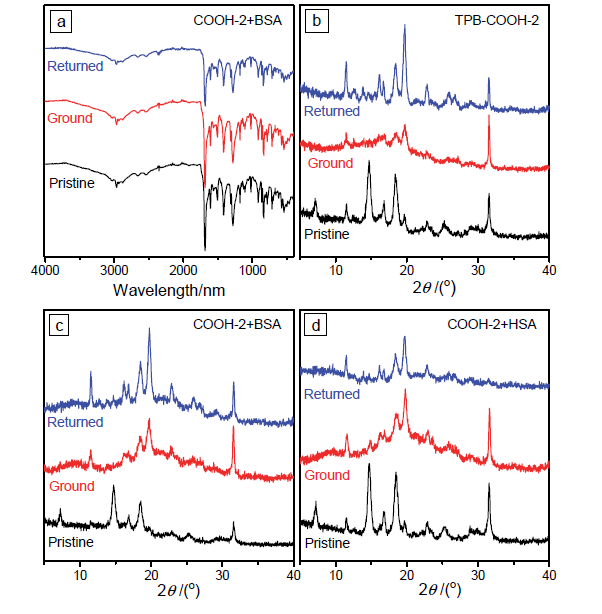
| [1] |
Luo, J.; Xie, Z.; Lam, J. W. Y.; Cheng, L.; Tang, B. Z.; Chen, H.; Qiu, C.; Kwok, H. S.; Zhan, X.; Liu, Y.; Zhu, D. Chem. Commun. 2001, (18), 1740.
|
| [2] |
Chen, Y.; Lam, J. W. Y.; Kwok, R. T. K.; Liu, B.; Tang, B. Z. Mater. Horiz. 2019, 6, 428.
doi: 10.1039/C8MH01331D |
| [3] |
Zhang, Y.; Mao, H.; Xu, W.; Shi, J.; Cai, Z.; Tong, B.; Dong, Y. Chem. Eur. J. 2018, 24, 15965.
doi: 10.1002/chem.201802114 |
| [4] |
Yuan, Y.; Zhang, C.-J.; Xu, S.; Liu, B. Chem. Sci. 2016, 7, 1862.
doi: 10.1039/C5SC03583J |
| [5] |
Gao, H.; Jiao, D.; Ou, H.; Zhang, J.; Ding, D. Acta Chim. Sinica 2021, 79, 319. (in Chinese)
doi: 10.6023/A20100501 |
|
(高贺麟, 焦迪, 欧翰林, 章经天, 丁丹, 化学学报, 2021, 79, 319.)
doi: 10.6023/A20100501 |
|
| [6] |
Shao, L.; Sun, J.; Hua, B.; Huang, F. Chem. Commun. 2018, 54, 4866.
doi: 10.1039/C8CC02077A |
| [7] |
Han, T.; Zhang, Y.; Feng, X.; Lin, Z.; Tong, B.; Shi, J.; Zhi, J.; Dong, Y. Chem. Commun. 2013, 49, 7049.
doi: 10.1039/c3cc42644k |
| [8] |
Zhang, Y.; Han, T.; Gu, S.; Zhou, T.; Zhao, C.; Guo, Y.; Feng, X.; Tong, B.; Bing, J.; Shi, J.; Zhi, J.; Dong, Y. Chem. Eur. J. 2014, 20, 8856.
|
| [9] |
Wang, Y.; Pan, X.; Peng, Z.; Zhang, Y.; Liu, P.; Cai, Z.; Tong, B.; Shi, J.; Dong, Y. Sens. Actuators B: Chem. 2018, 267, 351.
doi: 10.1016/j.snb.2018.04.056 |
| [10] |
Guo, Y.; Feng, X.; Han, T.; Wang, S.; Lin, Z.; Dong, Y.; Wang, B. J. Am. Chem. Soc. 2014, 136, 15485.
doi: 10.1021/ja508962m |
| [11] |
Chen, J.; Xia, J.; Gao, W.-J.; Yu, H.-J.; Zhong, J.-X.; Jia, C.; Qin, Y.-S.; She, Z.; Kuang, D.-B.; Shao, G. ACS Appl. Mater. Interfaces 2020, 12, 21088.
doi: 10.1021/acsami.0c02751 |
| [12] |
Guo, Y.; Gu, S.; Feng, X.; Wang, J.; Li, H.; Han, T.; Dong, Y.; Jiang, X.; James, T. D.; Wang, B. Chem. Sci. 2014, 5, 4388.
doi: 10.1039/C4SC01611D |
| [13] |
He, Z.; Liu, P.; Zhang, S.; Yan, J.; Wang, M.; Cai, Z.; Wang, J.; Dong, Y. Angew. Chem. Int. Ed. 2019, 58, 3834.
doi: 10.1002/anie.v58.12 |
| [14] |
Liu, P.; Chen, D.; Wang, Y.; Tang, X.; Li, H.; Shi, J.; Tong, B.; Dong, Y. Biosens. Bioelectron. 2017, 92, 536.
doi: 10.1016/j.bios.2016.10.064 |
| [15] |
Bera, M. K.; Chakraborty, C.; Malik, S. J. Mater. Chem. C 2017, 5, 6872.
doi: 10.1039/C6TC04906K |
| [16] |
Xu, Z.; Gu, J.; Qiao, X.; Qin, A.; Tang, B. Z.; Ma, D. ACS Photonics 2019, 6, 767.
doi: 10.1021/acsphotonics.8b01724 |
| [17] |
Zheng, K.; Ni, F.; Chen, Z.; Zhong, C.; Yang, C. Angew. Chem. Int. Ed. 2020, 59, 9972.
doi: 10.1002/anie.v59.25 |
| [18] |
Huang, G.; Jiang, Y.; Yang, S.; Li, B. S.; Tang, B. Z. Adv. Funct. Mater. 2019, 29, 1900516.
doi: 10.1002/adfm.v29.16 |
| [19] |
Li, W.; Huang, Q.; Mao, Z.; Li, Q.; Jiang, L.; Xie, Z.; Xu, R.; Yang, Z.; Zhao, J.; Yu, T.; Zhang, Y.; Aldred, M. P.; Chi, Z. Angew. Chem. Int. Ed. 2018, 57, 12727.
doi: 10.1002/anie.201806861 |
| [20] |
Liu, M.; Wu, Q.; Shi, H.; An, Z.; Huang, W. Acta Chim. Sinica 2018, 76, 246. (in Chinese)
doi: 10.6023/A17110504 |
|
(刘明丽, 吴琪, 史慧芳, 安众福, 黄维, 化学学报, 2018, 76, 246.)
doi: 10.6023/A17110504 |
|
| [21] |
Hu, M.; Feng, H.-T.; Yuan, Y.-X.; Zheng, Y.-S.; Tang, B. Z. Coordin. Chem. Rev. 2020, 416, 213329.
doi: 10.1016/j.ccr.2020.213329 |
| [22] |
Huang, Y.; You, X.; Wang, L.; Zhang, G.; Gui, S.; Jin, Y.; Zhao, R.; Zhang, D. Angew. Chem. Int. Ed. 2020, 59, 10042.
doi: 10.1002/anie.v59.25 |
| [23] |
Wang, J.; Chai, Z.; Wang, J.; Wang, C.; Han, M.; Liao, Q.; Huang, A.; Lin, P.; Li, C.; Li, Q.; Li, Z. Angew. Chem. Int. Ed. 2019, 58, 17297.
doi: 10.1002/anie.v58.48 |
| [24] |
Li, Q.; Li, Z. Acc. Chem. Res. 2020, 53, 962.
doi: 10.1021/acs.accounts.0c00060 |
| [25] |
Wang, J.; Li, Z. Acta Chim. Sinica 2021, 79, 575. (in Chinese)
doi: 10.6023/A21010029 |
|
(王金凤, 李振, 化学学报, 2021, 79, 575.)
doi: 10.6023/A21010029 |
|
| [26] |
Zhou, T.; Qian, Y.; Wang, H.; Feng, Q.; Xie, L.; Huang, W. Acta Chim. Sinica 2021, 79, 557. (in Chinese)
doi: 10.6023/A21010009 |
|
(周涛, 钱越, 王宏健, 冯全友, 解令海, 黄维, 化学学报, 2021, 79, 557.)
doi: 10.6023/A21010009 |
|
| [27] |
Liu, J.; Chen, C.; Ji, S.; Liu, Q.; Ding, D.; Zhao, D.; Liu, B. Chem. Sci. 2017, 8, 2782.
doi: 10.1039/C6SC04384D |
| [28] |
Qu, J.; Ren, F.; Shi, J.; Tong, B.; Cai, Z.; Dong, Y. Chem. Eur. J. 2020, 26, 14947.
doi: 10.1002/chem.v26.65 |
| [29] |
Li, N.; Liu, Y. Y.; Li, Y.; Zhuang, J. B.; Cui, R. R.; Gong, Q.; Zhao, N.; Tang, B. Z. ACS Appl. Mater. Interfaces 2018, 10, 24249.
doi: 10.1021/acsami.8b04113 |
| [30] |
Qiu, Z.; Yang, Z.; Chen, W.-C.; Xing, L.; Hu, S.; Ji, S.; Yang, Q.; Cai, N.; Ouyang, X.; Huo, Y. J. Mater. Chem. C 2020, 8, 4139.
doi: 10.1039/C9TC05864H |
| [31] |
Li, J.-H.; Liang, Y.; Xie, Y.-X. J. Org. Chem. 2004, 69, 8125.
doi: 10.1021/jo0486645 |
| [32] |
Li, Y.; Lei, Y.; Dong, L.; Zhang, L.; Zhi, J.; Shi, J.; Tong, B.; Dong, Y. Chem. Eur. J. 2019, 25, 573.
|
| [33] |
Yu, H.-X.; Zhi, J.; Wang, J.-L. J. Mater. Chem. C 2021, 9, 3882.
doi: 10.1039/D0TC05994C |
| [34] |
Zhang, L.; Liang, K.; Dong, L.; Yang, P.; Li, Y.; Feng, X.; Zhi, J.; Shi, J.; Tong, B.; Dong, Y. New J. Chem. 2017, 41, 8877.
doi: 10.1039/C7NJ00583K |
| [35] |
Dong, Y.; Zhang, J.; Li, A.; Gong, J.; He, B.; Xu, S.; Yin, J.; Liu, S. H.; Tang, B. Z. J. Mater. Chem. C 2020, 8, 894.
doi: 10.1039/C9TC06297A |
| [36] |
Xia, Z.; Shao, A.; Li, Q.; Zhu, S.; Zhu, W. Acta Chim. Sinica 2016, 74, 351. (in Chinese)
doi: 10.6023/A16010001 |
|
(夏志清, 邵安东, 李强, 朱世琴, 朱为宏, 化学学报, 2016, 74, 351.)
doi: 10.6023/A16010001 |
|
| [37] |
Zhang, X.; Zhang, Y.; Zhang, H.; Quan, Y.; Li, Y.; Cheng, Y.; Ye, S. Org. Lett. 2019, 21, 439.
doi: 10.1021/acs.orglett.8b03620 |
| [38] |
Peng, Z.; Ji, Y.; Huang, Z.; Tong, B.; Shi, J.; Dong, Y. Mater. Chem. Front. 2018, 2, 1175.
doi: 10.1039/C8QM00096D |
| [39] |
Feng, X.; Tong, B.; Shen, J.; Shi, J.; Han, T.; Chen, L.; Zhi, J.; Lu, P.; Ma, Y.; Dong, Y. J. Phys. Chem. B 2010, 114, 16731.
doi: 10.1021/jp108254g |
| [40] |
Gao, H.; Xu, D.; Wang, Y.; Zhang, C.; Yang, Y.; Liu, X.; Han, A.; Wang, Y. Dyes Pigments 2018, 150, 165.
doi: 10.1016/j.dyepig.2017.12.016 |
| [41] |
Lei, Y.; Lai, Y.; Dong, L.; Shang, G.; Cai, Z.; Shi, J.; Zhi, J.; Li, P.; Huang, X.; Tong, B.; Dong, Y. Chem. Eur. J. 2018, 24, 434.
doi: 10.1002/chem.v24.2 |
| [42] |
Song, W.; Zhi, J.; Wang, T.; Li, B.; Ni, S.; Ye, Y.; Wang, J. Chem. Asian J. 2019, 14, 3875.
doi: 10.1002/asia.v14.21 |
| [43] |
Ràfols, C.; Amézqueta, S.; Fuguet, E.; Bosch, E. J. Pharm. Biomed. Anal. 2018, 150, 452.
doi: S0731-7085(17)32363-4 pmid: 29291587 |
| [44] |
Li, M.; Zhang, J.; Liu, D.; Zhu, Y.; Zhang, Y. Chem. J. Chinese Univ. 2021, 42, 731. (in Chinese)
|
|
(李梦硕, 张静, 刘丹, 朱亚先, 张勇, 高等学校化学学报, 2021, 42, 731.)
|
| [1] | Zeng Chongyang, Hu Ping, Wang Biqin, Fang Wenyan, Zhao Keqing, Donnio Bertrand. Star-shaped Triphenylene-triazine Multi-stimuli Responsive Discotic Liquid Crystals: Synthesis, Properties and Applications [J]. Acta Chimica Sinica, 2023, 81(5): 469-479. |
| [2] | Bin Liu, Pangkuan Chen. Synthesis and Properties of Novel Circularly Polarized Luminescence Materials Based on Binaphthol Skeleton [J]. Acta Chimica Sinica, 2022, 80(7): 929-935. |
| [3] | Jinfeng Wang, Zhen Li. Significant Influence of Molecular Packing in Aggregates on Optoelectronic Properties [J]. Acta Chimica Sinica, 2021, 79(5): 575-587. |
| [4] | Heqi Gao, Di Jiao, Hanlin Ou, Jingtian Zhang, Dan Ding. High Performance Aggregation-Induced Emission Nanoprobes for Image-Guided Cancer Surgery [J]. Acta Chimica Sinica, 2021, 79(3): 319-325. |
| [5] | Guan Xiaolin, Li Zhifei, Wang Lin, Liu Meina, Wang Kailong, Yang Xueqin, Li Yali, Hu Lili, Zhao Xiaolong, Lai Shoujun, Lei Ziqiang. Preparation of AIE Polymer Dots (Pdots) Based on Poly(N-vinyl-2-pyrrolidone)-Eu(III) Complex and Dual-color Live Cell Imaging [J]. Acta Chimica Sinica, 2019, 77(12): 1268-1278. |
| [6] | Xia Zhiqing, Shao Andong, Li Qiang, Zhu Shiqin, Zhu Weihong. Substituent Effect on Quinoline-Malononitrile AIE Fluorescent Properties [J]. Acta Chim. Sinica, 2016, 74(4): 351-355. |
| [7] | Sun Jingbo, Zhang Gonghe, Jia Xiaoyu, Xue Pengchong, Jia Junhui, Lu Ran. Synthesis, Mechanochromism and Acid Response of the Fluorescence Dyes Based on Quinoxalines Modified with Tetraphenylethylenes [J]. Acta Chim. Sinica, 2016, 74(2): 165-171. |
| [8] | Peng Zhe, Ji Yingchun, Wang Zhi, Tong Bin, Shi Jianbing, Dong Yuping. Properties of Polymorphism and Acid Response of Pyrrolopyrrole-based Derivative with Aggregation-induced Emission Behavior [J]. Acta Chim. Sinica, 2016, 74(11): 942-948. |
| [9] | Han Ting, Cathy K. W. Jim, Jacky W. Y. Lam, Tang Ben Zhong. Polyynes with Aggregation-Induced Emission Characteristics: Synthesis and Their Photonic Properties [J]. Acta Chim. Sinica, 2016, 74(11): 877-884. |
| [10] | Bin Xin, Luo Weijian, Yuan Wangzhang, Zhang Yongming. Clustering-Triggered Emission of Poly(N-hydroxysuccinimide Methacrylate) [J]. Acta Chim. Sinica, 2016, 74(11): 935-941. |
| [11] | Bai Wei, Shi Yang, Song Chen, He Jie, Qin Anjun, Sun Jing Zhi, Tang Ben Zhong. Fluoranthene-Modified Tetraphenylethene Derivatives: Synthesis, Aggregation-Enhanced Emission Characteristic and Their Highly Sensitive Detection of Picric Acid [J]. Acta Chim. Sinica, 2016, 74(11): 893-901. |
| [12] | Guan Weijiang, Zhou Wenjuan, Lü Chao. Ultrathin Luminescence Film Based on Gold Nanoclusters with Aggregation-Induced Emission [J]. Acta Chim. Sinica, 2016, 74(11): 929-934. |
| [13] | Ji Guang, Yan Lulin, Wang Hui, Ma Lian, Xu Bin, Tian Wenjing. Efficient Near-infrared AIE Nanoparticles for Cell Imaging [J]. Acta Chim. Sinica, 2016, 74(11): 917-922. |
| [14] | Huang Yuzhang, Lei Luoqi, Zheng Chao, Wei Bo, Zhao Zujin, Qin Anjun, Hu Rongrong, Tang Ben Zhong. Tetraphenylethene-Containing Alkynone Derivatives: Design and Synthesis, Aggregation-Induced Emission Characteristics, and the Selective Fluorescence Detection of Pd2+ [J]. Acta Chim. Sinica, 2016, 74(11): 885-892. |
| [15] | Wang Zhi, Zhou Hongjun, Hu Junyi, You Jingsong, Gao Ge . Bisimidazole and Bisimidazolium Cruciforms: Synthesis and Discrimination of Organic Acids [J]. Acta Chimica Sinica, 2013, 71(9): 1257-1264. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||