Acta Chimica Sinica ›› 2022, Vol. 80 ›› Issue (4): 476-484.DOI: 10.6023/A21120603 Previous Articles Next Articles
Special Issue: 中国科学院青年创新促进会合辑
Article
刘俊瑞a,b,c, 陈晶琳d, 杨杰b,c, 许肖锋b,c, 李若男b,c, 黄有桂b,c, 陈少华d, 叶欣d,*( ), 王维b,c,*(
), 王维b,c,*( )
)
投稿日期:2021-12-30
发布日期:2022-04-28
通讯作者:
叶欣, 王维
作者简介:基金资助:
Junrui Liua,b,c, Jinglin Chend, Jie Yangb,c, Xiaofeng Xub,c, Ruonan Lib,c, You-Gui Huangb,c, Shaohua Chend, Xin Yed( ), Wei Wangb,c(
), Wei Wangb,c( )
)
Received:2021-12-30
Published:2022-04-28
Contact:
Xin Ye, Wei Wang
About author:Supported by:Share
Junrui Liu, Jinglin Chen, Jie Yang, Xiaofeng Xu, Ruonan Li, You-Gui Huang, Shaohua Chen, Xin Ye, Wei Wang. K+-Site Ce-Doped Jarosite for Phosphate Adsorption: a Mechanism Study※[J]. Acta Chimica Sinica, 2022, 80(4): 476-484.

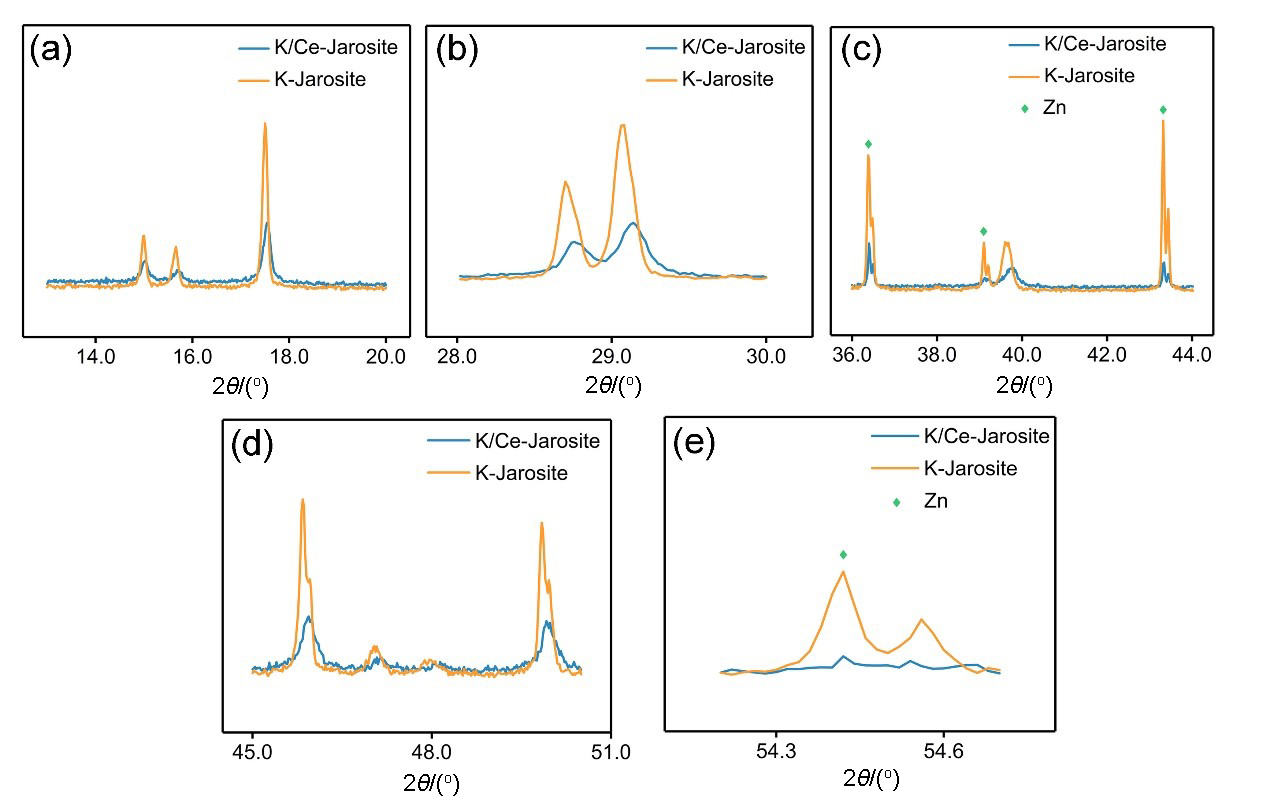
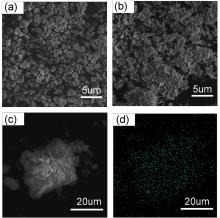
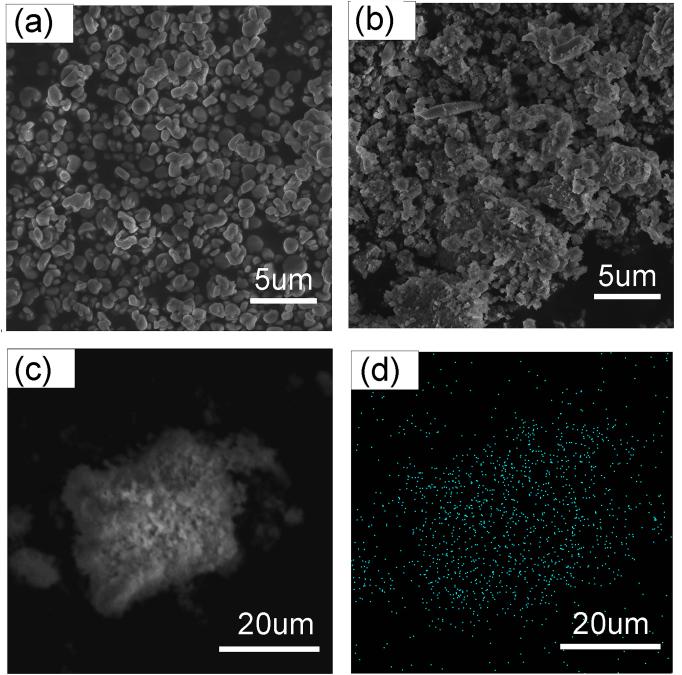
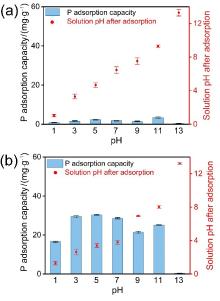


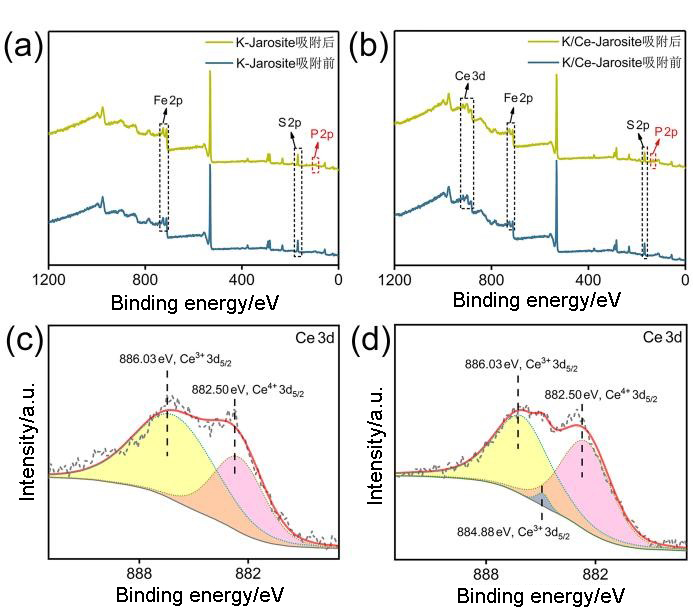
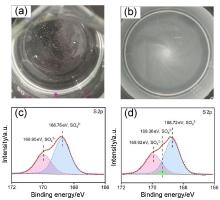
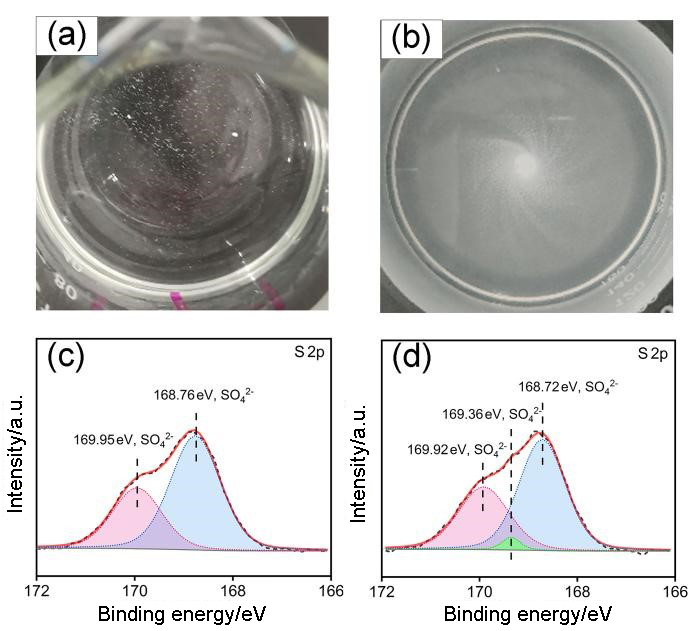
| [1] |
Strayer, D. L.; Dudgeon, D. J. N. Am. Benthol. Soc. 2010, 29, 344.
doi: 10.1899/08-171.1 |
| [2] |
Yang, X. H.; Cai, J. B.; Chen, L. H.; Cao, X.; Liu, H. Z.; Liu, M. X.; Chem. Eng. J. 2021, 425, 130623.
doi: 10.1016/j.cej.2021.130623 |
| [3] |
Zhang, Z.; Bi, X.; Li, X. T.; Zhao, Q. C.; Chen, H. H. RSC Adv. 2018, 8, 33583.
doi: 10.1039/C8RA06025H |
| [4] |
Eskandarpour, A.; Sassa, K.; Bando, Y.; Okido, M.; Asai, S. Mater. Trans. 2006, 47, 1837.
|
| [5] |
Wang, Y.; Wang, J. J.; Li, P.; Qin, H. B.; Liang, J. J.; Fan, Q. H. Environ. Technol. Innovation 2021, 23, 101615.
doi: 10.1016/j.eti.2021.101615 |
| [6] |
Elwood Madded, M. E.; Bodnar, R. J.; Rimstidt, J. D. Nature 2004, 431, 821.
doi: 10.1038/nature02971 |
| [7] |
Dutrizac, J. E.; Jambor, J. L. Rev. Mineral. Geochem. 2000, 40, 405.
doi: 10.2138/rmg.2000.40.8 |
| [8] |
Jin, X. H.; Li, X. F.; Guo, C.; Jiang, M. G.; Yao, Q.; Lu, G. N.; Dang, Z. Sci. Total Environ. 2020, 719, 137311.
doi: 10.1016/j.scitotenv.2020.137311 |
| [9] |
Karimian, N.; Johnston, S. G.; Burton, E. D. Environ. Sci. Technol. 2017, 51, 4259.
doi: 10.1021/acs.est.6b05335 |
| [10] |
Fan, C.; Guo, C. L.; Zeng, Y. F.; Tu, Z. H.; Ji, Y. P.; Reinfelder, J. R.; Chen, M. Q.; Huang, W. L.; Lu, G. N.; Yi, X. Y.; Dang, Z. Chemosphere 2019, 222, 945.
doi: 10.1016/j.chemosphere.2019.01.142 |
| [11] |
Tang, Y. G.; Xie, Y. Y.; Lu, G. N.; Ye, H.; Dang, Z.; Wen, Z. N.; Tao, X. Q.; Xie, C. S.; Yi, X. Y. Chemosphere 2020, 255, 126938.
doi: 10.1016/j.chemosphere.2020.126938 |
| [12] |
Li, H.; Wang, N. N.; Xiao, T. F.; Zhang, X. T.; Wang, J. Q.; Tang, J. F.; Kong, Q. N.; Fu, C. B.; Quan, H. B. Chemosphere 2021, 285, 131525.
doi: 10.1016/j.chemosphere.2021.131525 |
| [13] |
Dutrizac, J. E.; Chen, T. T. Hydrometallurgy 2010, 102, 5.
|
| [14] |
Mayakaduwage, S.; Mosley, L. M.; Marschner, P. Geoderma 2020, 371, 114359.
doi: 10.1016/j.geoderma.2020.114359 |
| [15] |
Liu, X. W.; Byrne, R. H. Geochim. Cosmochim. Acta 1997, 61, 1625.
doi: 10.1016/S0016-7037(97)00037-9 |
| [16] |
Gu, W.; Xie, Q.; Xing, M. C.; Wu, D. Y. Chem. Eng. Res. Des. 2017, 117, 706.
doi: 10.1016/j.cherd.2016.11.026 |
| [17] |
Hassan, M. H.; Stanton, R.; Secora, J.; Trivedi, D. J.; Andreescu, S. ACS Appl. Mater. Interfaces 2020, 12, 52788.
doi: 10.1021/acsami.0c16477 |
| [18] |
Neale, Z. G.; Barta, M.; Cao, G. Z. ACS Appl. Energ. Mater. 2021, 4, 2248.
|
| [19] |
Shannon, R. D. Acta Cryst. 1976, 32, 751.
doi: 10.1107/S0567739476001551 |
| [20] |
De Simone, C. A.; Mascarenhas, Y. P.; Svisero, D. P. Rev. Bras. Geocienc. 1985, 15, 164.
|
| [21] |
Spratt, H. J.; Rintoul, L.; Avdeev, M.; Martens, W. N. Am. Mineral. 2013, 98, 1633.
doi: 10.2138/am.2013.4486 |
| [22] |
Grohol, D.; Huang, Q. Z.; Toby, B. H.; Lynn, J. W.; Lee, Y. S.; Nocera, D. G. Phys. Rev. B 2003, 68, 94404.
doi: 10.1103/PhysRevB.68.094404 |
| [23] |
Grohol, D.; Nocera, D. G. J. Am. Chem. Soc. 2002, 124, 2640.
doi: 10.1021/ja016832u |
| [24] |
Basciano, L. C. Ph.D. Dissertation, Queen's University, Kingston, 2008.
|
| [25] |
Labib, S.; Abdelaal, S.; Abdelhady, A. M.; Elmaghraby, E. K. Mater. Chem. Phys. 2020, 256, 123654.
doi: 10.1016/j.matchemphys.2020.123654 |
| [26] |
Basciano, L. C.; Peterson, R. C. Am. Mineral. 2008, 93, 853.
doi: 10.2138/am.2008.2731 |
| [27] |
Hernández-Lazcano, E.; Cerecedo-Sáenz, E.; Hernández-Ávila, J.; Toro, N.; Karthik, T. V. K.; Mendoza-Anaya, D.; Fernández-García, M. E.; Rodríguez-Lugo, V.; Salinas-Rodríguez, E. Minerals 2021, 11, 80.
doi: 10.3390/min11010080 |
| [28] |
Zhang, Y.; Xi, X. Q.; Xu, S. N.; Zhou, J. C.; Zhou, J. J.; Xu, Q. H.; Shen, H. Y. Acta Chim. Sinica 2012, 70, 1839. (in Chinese)
doi: 10.6023/A12050171 |
|
(张蕴, 奚晓青, 许姗妮, 周俊晨, 周津金, 徐启宏, 沈昊宇, 化学学报, 2021, 70, 1839.)
|
|
| [29] |
Xie, J.; Wang, Z.; Fang, D.; Li, C. J.; Wu, D. Y. J. Colloid Interface Sci. 2014, 423, 13.
doi: 10.1016/j.jcis.2014.02.020 |
| [30] |
Trueman, A. M.; Fitzpatrick, R. W.; Mosley, L. M.; McLaughlin, M. J. Chem. Geol. 2021, 561, 120034.
doi: 10.1016/j.chemgeo.2020.120034 |
| [31] |
Xu, R.; Zhang, M. Y.; Mortimer, R. J.; Pan, G. Environ. Sci. Technol. 2017, 51, 3418.
doi: 10.1021/acs.est.6b05623 |
| [32] |
Ho, Y. S. Scientometrics 2004, 59, 171.
doi: 10.1023/B:SCIE.0000013305.99473.cf |
| [33] |
Ho, Y. S.; McKay, G. Process Biochem. 1999, 34, 451.
doi: 10.1016/S0032-9592(98)00112-5 |
| [34] |
Mohan, D.; Singh, K. P.; Singh, V. K. J. Hazard. Mater. 2006, 135, 280.
doi: 10.1016/j.jhazmat.2005.11.075 |
| [35] |
Ugurlu, M.; Kula, I.; Hamdi Karaoglu, M.; Arslan, Y. Environ. Prog. Sustainable Energy 2009, 28, 547.
doi: 10.1002/ep.10358 |
| [36] |
Bai, Z. A.; Chen, R. X.; Pang, H. W.; Wang, X. X.; Song, G.; Yu, S. J. Acta Chim. Sinica 2021, 79, 1265. (in Chinese)
doi: 10.6023/A21060263 |
|
(白子昂, 陈瑞兴, 庞宏伟, 王祥学, 宋刚, 于淑君, 化学学报, 2021, 79, 1265.)
doi: 10.6023/A21060263 |
|
| [37] |
Yang, A. L. Rare Met. Mater. Eng. 2018, 47, 1583. (in Chinese)
|
|
(杨爱丽, 稀有金属材料, 2018, 47, 1583.)
|
|
| [38] |
Haghseresht, F.; Lu, G. Q. Energ Fuels 1998, 12, 1100.
doi: 10.1021/ef9801165 |
| [39] |
Wang, G. Z.; Zeng, W.; Li, S. S. Environ. Sci. 2021, 42, 4815. (in Chinese)
|
|
(王光泽, 曾薇, 李帅帅, 环境科学, 2021, 42, 4815.)
|
|
| [40] |
Wang, F.; Liao, Q. L.; Chen, K. R.; Pan, S. Q.; Lu, M. W. J. Non-Cryst. Solids 2015, 409, 76.
doi: 10.1016/j.jnoncrysol.2014.11.020 |
| [41] |
Pemba-Mabiala, J. M.; Lenzi, M.; Lenzi, J.; Lebugle, A. Surf. Interface Anal. 1990, 15, 633.
|
| [42] |
Mao, D. S.; Luo, Z. H.; Li, Z. Y.; Hong, L.; Qu, R. F.; Wang, J. Q.; Jiang, L. J. Mol. Catal. (China) 2018, 32, 315. (in Chinese)
|
|
(冒德寿, 罗子豪, 李智宇, 洪鎏, 曲荣芬, 王家强, 姜亮, 分子催化, 2018, 32, 315.)
|
|
| [43] |
Kim, Y. J.; Wolf, A. S.; Becker, U. Geochim. Cosmochim. Acta 2019, 248, 138.
doi: 10.1016/j.gca.2018.11.017 |
| [44] |
Qi, P. F.; Pichler, T. J. Hazard. Mater. 2017, 330, 142.
doi: 10.1016/j.jhazmat.2017.02.016 |
| [45] |
Chen, K.; Jin, X. H.; Guo, C. L.; He, C. C.; Zhang, Y. Y.; Gao, K.; Lu, G. N.; Dang, Z. Chem. Geol. 2021, 579, 120338.
doi: 10.1016/j.chemgeo.2021.120338 |
| [46] |
Derycke, V.; Kongolo, M.; Benzaazoua, M.; Mallet, M.; Barrès, O.; De Donato, P.; Bussière, B.; Mermillod-Blondin, R. Int. J. Miner. Process. 2013, 118, 1.
doi: 10.1016/j.minpro.2012.10.004 |
| [47] |
Neal, A. L.; Techkarnjanaruk, S.; Dohnalkova, A.; Mccready, D.; Peyton, B. M.; Geesey, G. G. Geochim. Cosmochim. Acta 2001, 65, 223.
doi: 10.1016/S0016-7037(00)00537-8 |
| [48] |
Siriwardane, R. V.; Cook, J. M. J. Colloid Interface Sci. 1986, 114, 525.
doi: 10.1016/0021-9797(86)90438-8 |
| [49] |
Cao, L. N.; Chen, B. H.; Gou, X. Y.; Zou, Q. Geol. J. Chin. Univ. 2019, 25, 333. (in Chinese)
|
|
(曹丽娜, 陈炳辉, 苟习颖, 邹琦, 高等地质学报, 2019, 25, 333.)
|
|
| [50] |
Bi, S. F.; Cui, X. M. J. Salt. Chem. Ind. 2015, 44, 26. (in Chinese)
|
|
(毕思峰, 崔香梅, 盐业与化工, 2015, 44, 26.)
|
| [1] | Hangqing Lin, Ruoru Ma, Yilan Jiang, Murong Xu, Yangpeng Lin, Kezhao Du. Research Progress of Materials Used for Elemental Halogen Capture [J]. Acta Chimica Sinica, 2024, 82(1): 62-74. |
| [2] | Yuchun Han, Yilin Wang. Retrospect and Prospect of Long-lasting Antibacterial Materials★ [J]. Acta Chimica Sinica, 2023, 81(9): 1196-1201. |
| [3] | Kaiqing Wang, Shuo Yuan, Wangdong Xu, Dan Huo, Qiulin Yang, Qingxi Hou, Dehai Yu. Preparation and Adsorption Properties of ZIF-8@B-CNF Composite Aerogel [J]. Acta Chimica Sinica, 2023, 81(6): 604-612. |
| [4] | Ziqi Li, Liwei Liu, Chenghui Mao, Changkai Zhou, Minqi Xia, Zhen Shen, Yue Guo, Qiang Wu, Xizhang Wang, Lijun Yang, Zheng Hu. Cobalt-Substituted Polyoxometalates as Soluble Mediators to Boost the Lithium-Sulfur Battery Performance [J]. Acta Chimica Sinica, 2023, 81(6): 620-626. |
| [5] | Shaojuan Zeng, Xueqi Sun, Yinge Bai, Lu Bai, Shuang Zheng, Xiangping Zhang, Suojiang Zhang. Research Progress of CO2 Capture and Separation by Functionalized Ionic Liquids and Materials★ [J]. Acta Chimica Sinica, 2023, 81(6): 627-645. |
| [6] | Zhao Zhenxin, Yao Yikun, Chen Jiajun, Niu Rong, Wang Xiaomin. A High-entropy Phosphate Cathode Host towards High-stability Lithium-sulfur Batteries [J]. Acta Chimica Sinica, 2023, 81(5): 496-501. |
| [7] | Xu Yuanli, Pan Hui, Yang Yi, Zuo Zhiwei. Selectively Aerobic Oxidation of Benzylic C—H Bonds Enabled by Dual Anthracene and Cerium Catalysis under Continuous-Flow Conditions★ [J]. Acta Chimica Sinica, 2023, 81(5): 435-440. |
| [8] | Jiangmin Jiang, Xinran Zheng, Yating Meng, Wenjie He, Yaxin Chen, Quanchao Zhuang, Jiaren Yuan, Zhicheng Ju, Xiaogang Zhang. Research on the Preparation and Potassium Storage Performance of F, N Co-doped Porous Carbon Nanosheets [J]. Acta Chimica Sinica, 2023, 81(4): 319-327. |
| [9] | Wentao Wang, Xinting Lai, Shiquan Yan, Lei Zhu, Yuyuan Yao, Liming Ding. Synergistic Treatment of Dye Wastewater by the Adsorption-Degradation of a Bifunctional Aerogel [J]. Acta Chimica Sinica, 2023, 81(3): 222-230. |
| [10] | Bing Zheng, Zhe Wang, Jing He, Jiao Zhang, Wenbo Qi, Mengyuan Zhang, Haitao Yu. Structure and Work Function of Alkaline (Earth) Metal-Bilayer α-Borophene Nanocomposite: A Theoretical Study [J]. Acta Chimica Sinica, 2023, 81(10): 1357-1370. |
| [11] | Fang Liu, Tingting Pan, Xiurong Ren, Weiren Bao, Jiancheng Wang, Jiangliang Hu. Research on Preparation and Benzene Adsorption Performance of HCDs@MIL-100(Fe) Adsorbents [J]. Acta Chimica Sinica, 2022, 80(7): 879-887. |
| [12] | Tiantian Lü, Wen Ma, Dongsun Zhan, Yanmin Zou, Jilong Li, Meiling Feng, Xiaoying Huang. Two New Three-Dimensional Lanthanide Metal-organic Frameworks for the Highly Efficient Removal of Cs+ Ions※ [J]. Acta Chimica Sinica, 2022, 80(5): 640-646. |
| [13] | Yaru Wei, Jing Ma, Tingting Yuan, Jiawei Jiang, Yinli Duan, Juanqin Xue. Preparation and Adsorption Properties of Lithium Chloride Intercalation Carbon Nitride [J]. Acta Chimica Sinica, 2022, 80(4): 494-502. |
| [14] | Chaofeng Wang, Guodong Zheng, Yue Wang, Huijia Song, Xiaoyi Chen, Ruixia Gao. Preparation of Controllable Non-covalent Functionalized Carbon Nanotubes with Metalloporphyrin-Sn Network and Application to Protein Adsorption [J]. Acta Chimica Sinica, 2022, 80(2): 126-132. |
| [15] | Yarui Song, Kaisheng Wang, Guangyu An, Fajun Zhao, Bin Men, Zhaoxi Du, Dongsheng Wang. Preparation of Powdered Activated Carbon Matrix Composites and Their Decontamination Performance and Mechanisms for Oily Sewage [J]. Acta Chimica Sinica, 2022, 80(12): 1592-1599. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||