Abstract
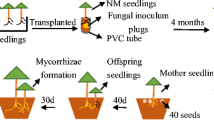
Many ectomycorrhizal (ECM) fungi produce commercially valuable edible sporocarps. However, the effects of nitrogen (N) application on ECM fungal sporocarp formation remain poorly understood. In this study, we investigated the effect of application of various N concentrations (0, 5, 25, 50, 100, and 200 mg/L) on the growth of Laccaria japonica mycelia in vitro for 1 month. The results showed that L. japonica mycelial biomass was highest in the 50 mg/L treatment and was significantly inhibited at N concentrations higher than 200 mg/L. Next, we investigated the effects of N application on mycorrhizal colonization and sporocarp formation in L. japonica colonizing Pinus densiflora seedlings in pots. The seedlings were watered with nutrient solutions containing 0, 5, 25, 50, or 100 mg N/L. The biomass, photosynthetic rate, and mycorrhizal colonization rates of the seedlings were measured at 45 days (first appearance of primordia), 65 days (sporocarp appearance on the substrate surface), and 4 months after seedlings were transplanted. The numbers of primordia and sporocarps were recorded during the experimental period. Total carbon (C) and N content were determined in seedlings at 4 months after transplantation, and in L. japonica sporocarps. Both mycelial growth and sporocarp production reached their maximum at an N application concentration of 50 mg/L, suggesting that the most suitable N concentration for ECM fungal sporocarp formation can easily be estimated in vitro during mycelial growth. This finding may help determine the most suitable N conditions for increasing edible ECM fungus sporocarp production in natural forests.






Similar content being viewed by others
References
Albarracín MV, Six J, Houlton BZ, Bledsoe CS (2013) A nitrogen fertilization field study of carbon-13 and nitrogen-15 transfers in ectomycorrhizas of Pinus sabiniana. Oecologia 173:1439–1450
Ambra R, Grimaldi B, Zamboni S, Filetici P, Macino G, Ballario P (2004) Photomorphogenesis in the hypogeous fungus Tuber borchii: isolation and characterization of Tbwc-1, the homologue of the blue-light photoreceptor of Neurospora crassa. Fungal Genet Biol 41:688–697
Arnebrant K (1994) Nitrogen amendments reduce the growth of extramatrical ectomycorrhizal mycelium. Mycorrhiza 5:7–15
Arnebrant K, Söderström B (1992) Effects of different fertilizer treatments on ectomycorrhizal colonization potential in two Scots pine forests in Sweden. For Ecol Manage 53:77–89
Aslani F, Tedersoo L, Põlme S, Knox O, Bahram M (2020) Global patterns and determinants of bacterial communities associated with ectomycorrhizal root tips of Alnus species. Soil Biol Biochem 148:107923
Corrales A, Turner BL, Tedersoo L, Anslan S, Dalling JW (2017) Nitrogen addition alters ectomycorrhizal fungal communities and soil enzyme activities in a tropical montane forest. Fungal Ecol 27:14–23
Cumming JR, Weinstein LH (1990) Aluminum-mycorrhizal interactions in the physiology of pitch pine seedlings. Plant Soil 125:7–18
Danielson RM, Griffiths CL, Parkinson D (1984) Effects of fertilization on the growth and mycorrhizal development of container-grown jack pine seedlings. Forest Science 30:828–835
Flores R, Bran MD, Honrubia M (2002) Edible mycorrhizal mushrooms of the west highland of Guatemala. In: Edible mycorrhizal mushrooms and their cultivation. Proceedings of the Second International Conference on Edible Mycorrhizal Mushrooms, Christchurch, New Zealand, 3–6 July, 2001, 2002. Crop & Food Research. pp 0–5
Frank HE, Garcia K (2021) Benefits provided by four ectomycorrhizal fungi to Pinus taeda under different external potassium availabilities. Mycorrhiza: 1–12
Franklin O, Näsholm T, Högberg P, Högberg MN (2014) Forests trapped in nitrogen limitation–an ecological market perspective on ectomycorrhizal symbiosis. New Phytol 203:657–666
Genre A, Lanfranco L, Perotto S, Bonfante P (2020) Unique and common traits in mycorrhizal symbioses. Nat Rev Microbiol 18:649–660
Godbout C, Fortin JA (1990) Cultural control of basidiome formation in Laccaria bicolor with container-grown white pine seedlings. Mycol Res 94:1051–1058
Godbout C, Fortin JA (1992) Effects of nitrogen fertilization and photoperiod on basidiome formation of Laccaria bicolor associated with container-grown jack pine seedlings. Can J Bot 70:181–185
Gorka S, Dietrich M, Mayerhofer W, Gabriel R, Wiesenbauer J, Martin V, Zheng Q, Imai B, Prommer J, Weidinger M, Schweiger P, Eichorst SA, Wagner M, Richter A, Schintlmeister A, Woebken D, Kaiser C (2019) Rapid transfer of plant photosynthates to soil bacteria via ectomycorrhizal hyphae and its interaction with nitrogen availability. Front Microbiol 10:168
Guerin-Laguette A (2021) Successes and challenges in the sustainable cultivation of edible mycorrhizal fungi–furthering the dream. Mycoscience 62:10–28
Hasselquist NJ, Högberg P (2014) Dosage and duration effects of nitrogen additions on ectomycorrhizal sporocarp production and functioning: an example from two N-limited boreal forests. Ecol Evol 4:3015–3026
Hasselquist NJ, Metcalfe DB, Högberg P (2012) Contrasting effects of low and high nitrogen additions on soil CO2 flux components and ectomycorrhizal fungal sporocarp production in a boreal forest. Glob Change Biol 18:3596–3605
Hobbie EA, Agerer R (2010) Nitrogen isotopes in ectomycorrhizal sporocarps correspond to belowground exploration types. Plant Soil 327(1):71–83
Hobbie EA, Chen J, Hasselquist NJ (2019) Fertilization alters nitrogen isotopes and concentrations in ectomycorrhizal fungi and soil in pine forests. Fungal Ecol 39:267–275
Högberg MN, Briones MJ, Keel SG, Metcalfe DB, Campbell C, Midwood AJ, Thornton B, Hurry V, Linder S, Näsholm T, Högberg P (2010) Quantification of effects of season and nitrogen supply on tree below-ground carbon transfer to ectomycorrhizal fungi and other soil organisms in a boreal pine forest. New Phytol 187:485–493
Högberg P, Nordgren A, Buchmann N, Taylor AF, Ekblad A, Högberg MN, Nyberg G, Ottosson-Löfvenius M, Read DJ (2001) Large-scale forest girdling shows that current photosynthesis drives soil respiration. Nature 411:789–792
Kaliyaperumal M, Kezo K, Gunaseelan S (2018) A Global Overview of Edible Mushrooms. In: Passari AK (ed) Singh BP, Lallawmsanga. Biology of Macrofungi. Springer International Publishing, Cham, pp 15–56
Karst J, Wasyliw J, Birch JD, Franklin J, Chang SX, Erbilgin N (2021) Long-term nitrogen addition does not sustain host tree stem radial growth but doubles the abundance of high-biomass ectomycorrhizal fungi. Glob Change Biol 27:4125–4138
Kennedy P (2010) Ectomycorrhizal fungi and interspecific competition: species interactions, community structure, coexistence mechanisms, and future research directions. New Phytol 187:895–910
Kjøller R, Nilsson LO, Hansen K, Schmidt IK, Vesterdal L, Gundersen P (2012) Dramatic changes in ectomycorrhizal community composition, root tip abundance and mycelial production along a stand-scale nitrogen deposition gradient. New Phytol 194:278–286
Kües U, Liu Y (2000) Fruiting body production in basidiomycetes. Appl Microbiol Biotechnol 54:141–152
Kuikka K, Härmä E, Markkola A, Rautio P, Roitto M, Saikkonen K, Ahonen-Jonnarth U, Finlay R, Tuomi J (2003) Severe defoliation of Scots pine reduces reproductive investment by ectomycorrhizal symbionts. Ecology 84:2051–2061
Lamhamedi MS, Godbout C, Fortin JA (1994) Dependence of Laccaria bicolor basidiome development on current photosynthesis of Pinus strobus seedlings. Can J for Res 24:1797–1804
Lilleskov EA, Fahey TJ, Lovett GM (2001) Ectomycorrhizal fungal aboveground community change over an atmospheric nitrogen deposition gradient. Ecol Appl 11:397–410
Lilleskov EA, Hobbie EA, Horton TR (2011) Conservation of ectomycorrhizal fungi: exploring the linkages between functional and taxonomic responses to anthropogenic N deposition. Fungal Ecol 4:174–183
Lilleskov EA, Hobbie EA, Fahey TJ (2002) Ectomycorrhizal fungal taxa differing in response to nitrogen deposition also differ in pure culture organic nitrogen use and natural abundance of nitrogen isotopes. New Phytol 154(1):219–231
Lilleskov EA, Kuyper TW, Bidartondo MI, Hobbie EA (2018) Atmospheric nitrogen deposition impacts on the structure and function of forest mycorrhizal communities: a review. Environ Pollut 246:148–162
Marx DH (1969) The influence of ectotrophic mycorrhizal fungi on the resistance of pine roots to pathogenic infections. I. Antagonism of mycorrhizal fungi to root pathogenic fungi and soil bacteria. Phytopathology 59:153–163
Marx DH, Hatch AB, Mendicino JF (1977) High soil fertility decreases sucrose content and susceptibility of loblolly pine roots to ectomycorrhizal infection by Pisolithus tinctorius. Can J Bot 55:1569–1574
Menge JA, Grand LF (1978) Effect of fertilization on production of epigeous basidiocarps by mycorrhizal fungi in loblolly pine plantations. Can J Bot 56:2357–2362
Nara K, Nakaya H, Hogetsu T (2003) Ectomycorrhizal sporocarp succession and production during early primary succession on Mount Fuji. New Phytol 158:193–206
Newton AC, Pigott CD (1991) Mineral nutrition and mycorrhizal infection of seedling oak and birch: I. Nutrient uptake and the development of mycorrhizal infection during seedling establishment. New Phytol 117:37–44
Nilsson LO, Wallander H (2003) Production of external mycelium by ectomycorrhizal fungi in a Norway spruce forest was reduced in response to nitrogen fertilization. New Phytol 158(2):409–416
Nylund JE (1988) The regulation of mycorrhiza formation-carbohydrate and hormone theories reviewed. Scand J for Res 3:465–479
Obase K (2020) Effects of bacterial strains isolated from the ectomycorrhizal roots of Laccaria parva on sporocarp production by the fungus in vitro. Mycoscience 61(1):9–15
Pena R, Polle A (2014) Attributing functions to ectomycorrhizal fungal identities in assemblages for nitrogen acquisition under stress. ISME J 8:321–330
R Core Team B (2018) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. Available at: https://www.R-project.org/. Version 3.5.1. Released July 2, 2018. Accessed 3 Oct 2018
Sakamoto Y (2018) Influences of environmental factors on fruiting body induction, development and maturation in mushroom-forming fungi. Fungal Biol Rev 32:236–248
Sharma R (2017) Ectomycorrhizal mushrooms: their diversity, ecology and practical applications. In: Mycorrhiza-Function, Diversity, State of the Art. Springer. pp 99–131
Smith SE, Read DJ (2008) Mycorrhizal symbiosis, 3rd edn. Academic press, New York, pp 191–268
Teramoto M, Wu B, Hogetsu T (2012) Transfer of 14 C-photosynthate to the sporocarp of an ectomycorrhizal fungus Laccaria amethystina. Mycorrhiza 22:219–225
Termorshuizen A, Ket PC (1991) Effects of ammonium and nitrate on mycorrhizal seedlings of Pinus sylvestris 1. Eur J for Pathol 21:404–413
Trappe MJ, Cromack K, Trappe JM, Wilson J, Rasmussen MC, Castellano MA, Miller SL (2009) Relationships of current and past anthropogenic disturbance to mycorrhizal sporocarp fruiting patterns at Crater Lake National Park, Oregon. Can J for Res 39:1662–1676
Velmala S (2014) Genetic control of susceptibility to fungal symbionts of juvenile Norway spruce (Picea abies (L.) H. Karsten) in relation to long-term growth performance. University of Helsinki, Finland
Victoroff C (2020) Response of ectomycorrhizal fungal fruiting to nitrogen and phosphorus additions in bartlett experimental forest, New Hampshire. Dissertations and Theses, State University of New York, New York
Vincenot L, Nara K, Sthultz C, Labbe J, Dubois M, Tedersoo L, Martin F, Selosse M (2012) Extensive gene flow over Europe and possible speciation over Eurasia in the ectomycorrhizal basidiomycete Laccaria amethystina complex. Mol Ecol 21:281–299
Vincenot L, Popa F, Laso F, Donges K, Rexer KH, Kost G, Yang ZL, Nara K, Selosse MA (2017) Out of Asia: Biogeography of fungal populations reveals Asian origin of diversification of the Laccaria amethystina complex, and two new species of violet Laccaria. Fungal Biol 121:939–955
Wallander H (1995) A new hypothesis to explain allocation of dry matter between mycorrhizal fungi and pine seedlings in relation to nutrient supply. In: Nilsson LO, Hüttl RF, Johansson UT (eds) Nutrient uptake and cycling in forest ecosystems. Springer, pp 243–248
Wallander H, Nylund JE (1992) Effects of excess nitrogen and phosphorus starvation on the extramatrical mycelium of ectomycorrhizas of Pinus sylvestris L. New Phytol 120:495–503
Wang T, Persson P, Tunlid A (2021) A widespread mechanism in ectomycorrhizal fungi to access nitrogen from mineral-associated proteins. Environ Microbiol 23(10):5837–5849
Wang Y, Cummings N, Guerin-Laguette A (2012) Cultivation of basidiomycete edible ectomycorrhizal mushrooms: Tricholoma, Lactarius, and Rhizopogon. In: Edible Ectomycorrhizal Mushrooms. Springer. pp 281–304
Wang Y, Hall IR, Evans LA (1997) Ectomycorrhizal fungi with edible fruiting bodies 1. Tricholoma matsutake and related fungi. Econ Bot 51:311–327
Wilson AW, Hosaka K, Mueller GM (2017) Evolution of ectomycorrhizas as a driver of diversification and biogeographic patterns in the model mycorrhizal mushroom genus Laccaria. New Phytol 213:1862–1873
Zhang J, Li T, Yang Y-L, Liu H-G, Wang Y-Z (2015) Arsenic concentrations and associated health risks in Laccaria mushrooms from Yunnan (SW China). Biol Trace Elem Res 164(2):261–266
Zhang S, Vaario L-M, Xia Y, Matsushita N, Geng Q, Tsuruta M, Kurokochi H, Lian C (2019) The effects of co-colonising ectomycorrhizal fungi on mycorrhizal colonisation and sporocarp formation in Laccaria japonica colonising seedlings of Pinus densiflora. Mycorrhiza 29:207–218
Acknowledgements
We are grateful to Mitsuko Goto, Yang Liu, Suguru Tanaka, Masayuki Kubota, Huayong Wang for technical assistance, the staff of the University of Tokyo Tanashi Forest for providing the forest soils, Kazuhide Nara for providing the culture of ectomycorrhizal fungus and Maki Narimatsu for providing the seeds of P. densiflora. We thank two anonymous reviewers for revising and useful comments on the manuscript.
Funding
This work was supported in part by JSPS KAKENHI (17H03824).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhang, S., Tsuruta, M., Li, C. et al. Estimation of the most suitable nitrogen concentration for sporocarp formation in Laccaria japonica colonizing Pinus densiflora seedlings through in vitro mycelial culture. Mycorrhiza 32, 451–464 (2022). https://doi.org/10.1007/s00572-022-01085-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00572-022-01085-2