Abstract
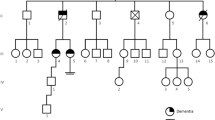
ATL1-related spastic paraplegia SPG3A is a pure form of hereditary spastic paraplegia. Rare complex phenotypes have been described, but few data concerning cognitive evaluation or molecular imaging of these patients are available. We relate a retrospective collection of patients with SPG3A from the Neurology Department of Nancy University Hospital, France. For each patient were carried out a 18F-FDG PET (positron emission tomography), a electromyography (EMG), a sudoscan®, a cerebral and spinal cord MRI (magnetic resonance imaging) with measurement of cervical and thoracic surfaces, a neuropsychological assessment. The present report outlines standardised clinical and paraclinical data of five patients from two east-France families carrying the same missense pathogenic variation, NM_015915.4(ATL1): c.1483C > T p.(Arg495Trp) in ATL1. Mean age at onset was 14 ± 15.01 years. Semi-quantitatively and in comparison to healthy age-matched subjects, PET scans showed a significant cerebellar and upper or mild temporal hypometabolism in all four adult patients and hypometabolism of the prefrontal cortex or precuneus in three of them. Sudoscan® showed signs of small fibre neuropathy in three patients. Cervical and thoracic patients’ spinal cords were significantly thinner than matched-control, respectively 71 ± 6.59mm2 (p = 0.01) and 35.64 ± 4.35mm2 (p = 0.015). Two patients presented with a dysexecutive syndrome. While adding new clinical and paraclinical signs associated with ATL1 pathogenic variations, we insist here on the variable penetrance and expressivity. We report small fibre neuropathy, cerebellar hypometabolism and dysexecutive syndromes associated with SPG3A. These cognitive impairments and PET findings may be related to a cortico-cerebellar bundle axonopathy described in the cerebellar cognitive affective syndrome (CCAS).





Similar content being viewed by others
References
Shribman S, Reid E, Crosby AH, Houlden H, Warner TT (2019) Hereditary spastic paraplegia: from diagnosis to emerging therapeutic approaches. Lancet Neurol 18:1136–1146. https://doi.org/10.1016/S1474-4422(19)30235-2
FaraziFard MA, Rebelo AP, Buglo E, Nemati H, Dastsooz H, Gehweiler I, Reich S, Reichbauer J, Quintáns B, Ordóñez-Ugalde A, Cortese A, Courel S, Abreu L, Powell E, Danzi MC, Martuscelli NB, Bis-Brewer DM, Tao F, Zarei F, Habibzadeh P, Yavarian M, Modarresi F, Silawi M, Tabatabaei Z, Yousefi M, Farpour HR, Kessler C, Mangold E, Kobeleva X, Tournev I, Chamova T, Mueller AJ, Haack TB, Tarnopolsky M, Gan-Or Z, Rouleau GA, Synofzik M, Sobrido M-J, Jordanova A, Schüle R, Zuchner S, Faghihi MA (2019) Truncating mutations in UBAP1 Cause hereditary spastic paraplegia. Am J Human Genet 104:767–773. https://doi.org/10.1016/j.ajhg.2019.03.001
Ahmed MY, Al-Khayat A, Al-Murshedi F, Al-Futaisi A, Chioza BA, Pedro Fernandez-Murray J, Self JE, Salter CG, Harlalka GV, Rawlins LE, Al-Zuhaibi S, Al-Azri F, Al-Rashdi F, Cazenave-Gassiot A, Wenk MR, Al-Salmi F, Patton MA, Silver DL, Baple EL, McMaster CR, Crosby AH (2017) A mutation of EPT1 (SELENOI) underlies a new disorder of Kennedy pathway phospholipid biosynthesis. Brain 140:547–554. https://doi.org/10.1093/brain/aww318
Vaz FM, McDermott JH, Alders M, Wortmann SB, Kölker S, Pras-Raves ML, Vervaart MAT, van Lenthe H, Luyf ACM, Elfrink HL, Metcalfe K, Cuvertino S, Clayton PE, Yarwood R, Lowe MP, Lovell S, Rogers RC, Study DDD, van Kampen AHC, Ruiter JPN, Wanders RJA, Ferdinandusse S, van Weeghel M, Engelen M, Banka S (2019) Mutations in PCYT2 disrupt etherlipid biosynthesis and cause a complex hereditary spastic paraplegia. Brain 142:3382–3397. https://doi.org/10.1093/brain/awz291
Husain RA, Grimmel M, Wagner M, Hennings JC, Marx C, Feichtinger RG, Saadi A, Rostásy K, Radelfahr F, Bevot A, Döbler-Neumann M, Hartmann H, Colleaux L, Cordts I, Kobeleva X, Darvish H, Bakhtiari S, Kruer MC, Besse A, Ng AC-H, Chiang D, Bolduc F, Tafakhori A, Mane S, GhasemiFirouzabadi S, Huebner AK, Buchert R, Beck-Woedl S, Müller AJ, Laugwitz L, Nägele T, Wang Z-Q, Strom TM, Sturm M, Meitinger T, Klockgether T, Riess O, Klopstock T, Brandl U, Hübner CA, Deschauer M, Mayr JA, Bonnen PE, Krägeloh-Mann I, Wortmann SB, Haack TB (2020) Bi-allelic HPDL variants cause a neurodegenerative disease ranging from neonatal encephalopathy to adolescent-onset spastic paraplegia. Am J Human Genet 107:364–373. https://doi.org/10.1016/j.ajhg.2020.06.015
Nielsen JE, Johnsen B, Koefoed P, Scheuer KH, Grønbech-Jensen M, Law I, Krabbe K, Nørremølle A, Eiberg H, Søndergård H, Dam M, Rehfeld JF, Krarup C, Paulson OB, Hasholt L, Sørensen SA (2004) Hereditary spastic paraplegia with cerebellar ataxia: a complex phenotype associated with a new SPG4 gene mutation. Eur J Neurol 11:817–824. https://doi.org/10.1111/j.1468-1331.2004.00888.x
Erfanian Omidvar M, Torkamandi S, Rezaei S, Alipoor B, Omrani MD, Darvish H, Ghaedi H (2019) Genotype-phenotype associations in hereditary spastic paraplegia: a systematic review and meta-analysis on 13,570 patients. J Neurol https://doi.org/10.1007/s00415-019-09633-1.
Zhao G, Liu X (2017) Clinical features and genotype-phenotype correlation analysis in patients with ATL1 mutations: A literature reanalysis. Transl Neurodegener 6. https://doi.org/10.1186/s40035-017-0079-3.
Zhao X, Alvarado D, Rainier S, Lemons R, Hedera P, Weber CH, Tukel T, Apak M, Heiman-Patterson T, Ming L, Bui M, Fink JK (2001) Mutations in a newly identified GTPase gene cause autosomal dominant hereditary spastic paraplegia. Nat Genet 29:326–331. https://doi.org/10.1038/ng758
Park SH, Zhu P-P, Parker RL, Blackstone C (2010) Hereditary spastic paraplegia proteins REEP1, spastin, and atlastin-1 coordinate microtubule interactions with the tubular ER network. J Clin Invest 120:1097–1110. https://doi.org/10.1172/JCI40979
Mészárosová AU, Grečmalová D, Brázdilová M, Dvořáčková N, Kalina Z, Čermáková M, Vávrová D, Smetanová I, Staněk D, Seeman P (2017) Disease-causing variants in the ATL1 gene are a rare cause of hereditary spastic paraplegia among Czech patients. Ann Hum Genet 81:249–257. https://doi.org/10.1111/ahg.12206
Ming L (2007) SPG3A-hereditary spastin paraplegia with genetic anticipation and incomplete penetrance. Zhonghua Yi Xue Yi Chuan Xue Za Zhi 24:15–18
Luo Y, Chen C, Zhan Z, Wang Y, Du J, Hu Z, Liao X, Zhao G, Wang J, Yan X, Jiang H, Pan Q, Xia K, Tang B, Shen L (2014) Mutation and clinical characteristics of autosomal-dominant hereditary spastic paraplegias in China. Neurodegener Dis 14:176–183. https://doi.org/10.1159/000365513
Ivanova N, Claeys KG, Deconinck T, Litvinenko I, Jordanova A, Auer-Grumbach M, Haberlova J, Löfgren A, Smeyers G, Nelis E, Mercelis R, Plecko B, Priller J, Zámecník J, Ceulemans B, Erichsen AK, Björck E, Nicholson G, Sereda MW, Seeman P, Kremensky I, Mitev V, De Jonghe P (2007) Hereditary spastic paraplegia 3A associated with axonal neuropathy. Arch Neurol 64:706–713. https://doi.org/10.1001/archneur.64.5.706
Schwartzlow C, Kazamel M (2019) Hereditary sensory and autonomic neuropathies: adding more to the classification. Curr Neurol Neurosci Rep 19:52. https://doi.org/10.1007/s11910-019-0974-3
Jacinto-Scudeiro LA, Dariva Machado G, Ayres A, Burguêz D, Polese-Bonato M, González-Salazar C, Siebert M, Cavalcante França Jr. M, Olchik MR, Morales Saute JA (2019) Are cognitive changes in hereditary spastic paraplegias restricted to complicated forms?. Front Neurol 10. https://doi.org/10.3389/fneur.2019.00508.
Chamard L, Ferreira S, Pijoff A, Silvestre M, Berger E, Magnin E (2016) Cognitive Impairment Involving Social Cognition in SPG4 Hereditary Spastic Paraplegia. Behav Neurol 2016:6423461. https://doi.org/10.1155/2016/6423461
da Graça FF, de Rezende TJR, Vasconcellos LFR, Pedroso JL, Barsottini OGP, França MC (2018) Neuroimaging in hereditary spastic paraplegias: current use and future perspectives. Front Neurol 9:1117. https://doi.org/10.3389/fneur.2018.01117
Morais S, Raymond L, Mairey M, Coutinho P, Brandão E, Ribeiro P, Loureiro JL, Sequeiros J, Brice A, Alonso I, Stevanin G (2017) Massive sequencing of 70 genes reveals a myriad of missing genes or mechanisms to be uncovered in hereditary spastic paraplegias. Eur J Hum Genet 25:1217–1228. https://doi.org/10.1038/ejhg.2017.124
Trummer B, Haubenberger D, Blackstone C (2018) Clinical trial designs and measures in hereditary spastic paraplegias. Front Neurol 9. https://doi.org/10.3389/fneur.2018.01017.
Rabin R, de Charro F (2001) EQ-5D: a measure of health status from the EuroQol Group. Ann Med 33:337–343. https://doi.org/10.3109/07853890109002087
Varrone A, Asenbaum S, Vander Borght T, Booij J, Nobili F, Någren K, Darcourt J, Kapucu OL, Tatsch K, Bartenstein P, Van Laere K, European Association of Nuclear Medicine Neuroimaging Committee (2009) EANM procedure guidelines for PET brain imaging using [18F]FDG, version 2. Eur J Nucl Med Mol Imaging 36:2103–2110. https://doi.org/10.1007/s00259-009-1264-0
Nasreddine ZS, Phillips NA, Bédirian V, Charbonneau S, Whitehead V, Collin I, Cummings JL, Chertkow H (2005) The Montreal cognitive assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc 53:695–699. https://doi.org/10.1111/j.1532-5415.2005.53221.x
Buschke H (1984) Cued recall in amnesia. J Clin Neuropsychol 6:433–440. https://doi.org/10.1080/01688638408401233
Grober E, Buschke H, Crystal H, Bang S, Dresner R (1988) Screening for dementia by memory testing. Neurology 38:900–903. https://doi.org/10.1212/wnl.38.6.900
Benedict RHB, Schretlen D, Groninger L, Dobraski M, Shpritz B (1996) Revision of the brief visuospatial memory test: studies of normal performance, reliability, and validity. Psychol Assess 8:145–153. https://doi.org/10.1037/1040-3590.8.2.145
Benton AL, Varney NR, deSHamsher K (1978) Visuospatial judgment: a clinical test. Arch Neurol 35:364–367. https://doi.org/10.1001/archneur.1978.00500300038006
Deloche G, Hannequin D, Dordain M, Metz-Lutz MN, Kremin H, Tessier C, Vendrell J, Cardebat D, Perrier D, Quint S, Pichard B (1997) Diversity of patterns of improvement in confrontation naming rehabilitation: some tentative hypotheses. J Commun Disord 30:11–21; quiz 21–22. https://doi.org/10.1016/0021-9924(95)00054-2.
Mahieux-Laurent F, Fabre C, Galbrun E, Dubrulle A, Moroni C (2009) Validation d’une batterie brève d’évaluation des praxies gestuelles pour consultation mémoire. évaluation chez 419 témoins, 127 patients atteints de troubles cognitifs légers et 320 patients atteints d’une démence. [Validation of a brief screening scale evaluating praxic abilities for use in memory clinics. Evaluation in 419 controls, 127 mild cognitive impairment and 320 demented patients]. Revue Neurologique 165:560–567. https://doi.org/10.1016/j.neurol.2008.11.016
Dubois B, Slachevsky A, Litvan I, Pillon B (2000) The FAB: a frontal assessment battery at bedside. Neurology 55:1621–1626. https://doi.org/10.1212/wnl.55.11.1621
Llinàs-Reglà J, Vilalta-Franch J, López-Pousa S, Calvó-Perxas L, Torrents Rodas D, Garre-Olmo J (2017) The Trail Making Test. Assessment 24:183–196. https://doi.org/10.1177/1073191115602552
Periáñez JA, Lubrini G, García-Gutiérrez A, Ríos-Lago M (2021) Construct validity of the stroop color-word test: influence of speed of visual search, verbal fluency, working memory, cognitive flexibility, and conflict monitoring. Arch Clin Neuropsychol 36:99–111. https://doi.org/10.1093/arclin/acaa034
TY - CHAP AU - Valentine, Thomas AU - Block, Cady AU - Eversole, Kara AU - Boxley, Laura AU - Dawson, Erica PY - 2020/09/18 SP - SN - 9781118970744 T1 - Wechsler Adult Intelligence Scale-IV (WAIS-IV) ER -
TY - BOOK AU - Godefroy, Olivier AU - GREFEX PY - 2008/01/01 SP - T1 - Fonctions exécutives et pathologies neurologiques et psychiatriques ER -
The SEA (Social cognition and Emotional Assessment): a clinical neuropsychological tool for early diagnosis of frontal variant of frontotemporal lobar degeneration - PubMed, (n.d.). https://pubmed-ncbi-nlm-nih-gov.bases-doc.univ-lorraine.fr/21895376/ (accessed October 17, 2020).
Bertoux M, Delavest M, de Souza LC, Funkiewiez A, Lépine JP, Fossati P, Dubois B, Sarazin M (2012) Social Cognition and Emotional Assessment differentiates frontotemporal dementia from depression. J Neurol Neurosurg Psychiatry 83(4):411–6. https://doi.org/10.1136/jnnp-2011-301849
Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J (1961) An inventory for measuring depression. Arch Gen Psychiatry 4:561–571. https://doi.org/10.1001/archpsyc.1961.01710120031004
Vinik AI, Smith AG, Singleton JR, Callaghan B, Freedman BI, Tuomilehto J, Bordier L, Bauduceau B, Roche F (2016) Normative values for electrochemical skin conductances and impact of ethnicity on quantitative assessment of sudomotor function. Diabetes Technol Ther 18:391–398. https://doi.org/10.1089/dia.2015.0396
Mustafa MI, Murshed NS, Abdelmoneim AH, Abdelmageed MI, Elfadol NM, Makhawi AM (2020) Extensive In Silico Analysis of ATL1 Gene : Discovered Five Mutations That May Cause Hereditary Spastic Paraplegia Type 3A. Scientifica (Cairo) 2020:8329286. https://doi.org/10.1155/2020/8329286
Dürr A, Camuzat A, Colin E, Tallaksen C, Hannequin D, Coutinho P, Fontaine B, Rossi A, Gil R, Rousselle C, Ruberg M, Stevanin G, Brice A (2004) Atlastin1 mutations are frequent in young-onset autosomal dominant spastic paraplegia. Arch Neurol 61:1867–1872. https://doi.org/10.1001/archneur.61.12.1867
Yan Y-T, Wei Q, Zheng Y, Luo W-J, Dong H-L, Lu C, Zhang J, Chen M-J, Bao Y-X, Li H-F (2019) Clinical and genetic characterization of a cohort of Chinese patients with hereditary spastic paraplegia. Clin Genet 95:637–639. https://doi.org/10.1111/cge.13517
Scheuer KH, Nielsen JE, Krabbe K, Simonsen C, Koefoed P, Sørensen SA, Gade A, Paulson OB, Law I (2005) Reduced regional cerebral blood flow in SPG4-linked hereditary spastic paraplegia. J Neurol Sci 235:23–32. https://doi.org/10.1016/j.jns.2005.03.051
Terada T, Kono S, Ouchi Y, Yoshida K, Hamaya Y, Kanaoka S, Miyajima H (2013) SPG3A-linked hereditary spastic paraplegia associated with cerebral glucose hypometabolism. Ann Nucl Med 27:303–308. https://doi.org/10.1007/s12149-012-0673-5
Faber I, Branco LMT, FrançaJúnior MC (2016) Cognitive dysfunction in hereditary spastic paraplegias and other motor neuron disorders. Dement Neuropsychol 10:276–279. https://doi.org/10.1590/s1980-5764-2016dn1004004
Zhu P-P, Soderblom C, Tao-Cheng J-H, Stadler J, Blackstone C (2006) SPG3A protein atlastin-1 is enriched in growth cones and promotes axon elongation during neuronal development. Hum Mol Genet 15:1343–1353. https://doi.org/10.1093/hmg/ddl054
Yalçın B, Zhao L, Stofanko M, O’Sullivan NC, Kang ZH, Roost A, Thomas MR, Zaessinger S, Blard O, Patto AL, Sohail A, Baena V, Terasaki M, O’Kane CJ (2017) Modeling of axonal endoplasmic reticulum network by spastic paraplegia proteins. Elife 6:e23882. https://doi.org/10.7554/eLife.23882
Orso G, Pendin D, Liu S, Tosetto J, Moss TJ, Faust JE, Micaroni M, Egorova A, Martinuzzi A, McNew JA, Daga A (2009) Homotypic fusion of ER membranes requires the dynamin-like GTPase Atlastin. Nature 460:978–983. https://doi.org/10.1038/nature08280
Liu X, Guo X, Niu L, Li X, Sun F, Hu J, Wang X, Shen K (2019) Atlastin-1 regulates morphology and function of endoplasmic reticulum in dendrites. Nat Commun 10:568. https://doi.org/10.1038/s41467-019-08478-6
Sanderson CM, Connell JW, Edwards TL, Bright NA, Duley S, Thompson A, Luzio JP, Reid E (2006) Spastin and atlastin, two proteins mutated in autosomal dominant hereditary spastic paraplegia, are binding partners. Hum Mol Genet 15:307–318. https://doi.org/10.1093/hmg/ddi447
Zhu P-P, Patterson A, Lavoie B, Stadler J, Shoeb M, Patel R, Blackstone C (2003) Cellular localization, oligomerization, and membrane association of the hereditary spastic paraplegia 3A (SPG3A) protein atlastin. J Biol Chem 278:49063–49071. https://doi.org/10.1074/jbc.M306702200
Schmahmann JD, Sherman JC (1998) The cerebellar cognitive affective syndrome. Brain 121(Pt 4):561–579. https://doi.org/10.1093/brain/121.4.561
Krienen FM, Buckner RL (2009) Segregated fronto-cerebellar circuits revealed by intrinsic functional connectivity. Cereb Cortex 19:2485–2497. https://doi.org/10.1093/cercor/bhp135
Hoche F, Guell X, Vangel MG, Sherman JC, Schmahmann JD (2018) The cerebellar cognitive affective/Schmahmann syndrome scale. Brain 141:248–270. https://doi.org/10.1093/brain/awx317
Mariën P, Ackermann H, Adamaszek M, Barwood CHS, Beaton A, Desmond J, De Witte E, Fawcett AJ, Hertrich I, Küper M, Leggio M, Marvel C, Molinari M, Murdoch BE, Nicolson RI, Schmahmann JD, Stoodley CJ, Thürling M, Timmann D, Wouters E, Ziegler W (2014) Consensus paper: language and the cerebellum: an ongoing enigma. Cerebellum 13:386–410. https://doi.org/10.1007/s12311-013-0540-5
Al-Maawali A, Rolfs A, Klingenhaeger M, Yoon G (2011) Hereditary spastic paraplegia associated with axonal neuropathy: a novel mutation of SPG3A in a large family. J Clin Neuromuscul Dis 12:143–146. https://doi.org/10.1097/CND.0b013e318209efc6
Fusco C, Frattini D, Farnetti E, Nicoli D, Casali B, Fiorentino F, Nuccitelli A, Giustina ED (2010) Hereditary spastic paraplegia and axonal motor neuropathy caused by a novel SPG3A de novo mutation. Brain Develop 32:592–594. https://doi.org/10.1016/j.braindev.2009.08.003
Scarano V, Mancini P, Criscuolo C, De Michele G, Rinaldi C, Tucci T, Tessa A, Santorelli FM, Perretti A, Santoro L, Filla A (2005) The R495W mutation in SPG3A causes spastic paraplegia associated with axonal neuropathy. J Neurol 252:901–903. https://doi.org/10.1007/s00415-005-0768-1
Fabry V, Gerdelat A, Acket B, Cintas P, Rousseau V, Uro-Coste E, Evrard SM, Pavy-Le Traon A. Which Method for Diagnosing Small Fiber Neuropathy? Front Neurol 11:342. https://doi.org/10.3389/fneur.2020.00342
Hedera P, Eldevik OP, Maly P, Rainier S, Fink JK (2005) Spinal cord magnetic resonance imaging in autosomal dominant hereditary spastic paraplegia. Neuroradiology 47:730–734. https://doi.org/10.1007/s00234-005-1415-3
Varga RE, Schüle R, Fadel H, Valenzuela I, Speziani F, Gonzalez M, Rudenskaia G, Nürnberg G, Thiele H, Altmüller J, Alvarez V, Gamez J, Garbern JY, Nürnberg P, Zuchner S, Beetz C (2013) Do not trust the pedigree: reduced and sex-dependent penetrance at a novel mutation hotspot in ATL1 blurs autosomal dominant inheritance of spastic paraplegia. Hum Muta 34(6):860–3. https://doi.org/10.1002/humu.22309
Cummings BB, Marshall JL, Tukiainen T, Lek M, Donkervoort S, Foley AR, Bolduc V, Waddell LB, Sandaradura SA, O'Grady GL, Estrella E, Reddy HM, Zhao F, Weisburd B, Karczewski KJ, O'Donnell-Luria AH, Birnbaum D, Sarkozy A, Hu Y, Gonorazky H, Claeys K, Joshi H, Bournazos A, Oates EC, Ghaoui R, Davis MR, Laing NG, Topf A (2017) Genotype-Tissue Expression Consortium, Kang PB, Beggs AH, North KN, Straub V, Dowling JJ, Muntoni F, Clarke NF, Cooper ST, Bönnemann CG, MacArthur DG. Improving genetic diagnosis in Mendelian disease with transcriptome sequencing. Sci Transl Med 9(386):eaal5209. https://doi.org/10.1126/scitranslmed.aal5209
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval
The study was approved by the local ethics committee.
Consent to participate
Written informed consent was obtained from all individuals contributing a blood sample for molecular investigations and the local ethics committees approved the study. A distinct consent form was also signed if videos were recorded.
Consent for publication
The final manuscript has been read and approved by all authors who accepted full responsibility for the design and undertaking of the original article. They had access to the data and co ntrolled the decision to publish.
Conflicts of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary file2 (MP4 7354 KB)
Supplementary file3 (MP4 5787 KB)
Appendix
Rights and permissions
About this article
Cite this article
Hocquel, A., Ravel, JM., Lambert, L. et al. Reduced penetrance of an eastern French mutation in ATL1 autosomal-dominant inheritance (SPG3A): extended phenotypic spectrum coupled with brain 18F-FDG PET. Neurogenetics 23, 241–255 (2022). https://doi.org/10.1007/s10048-022-00695-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10048-022-00695-4