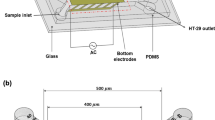
Abstract
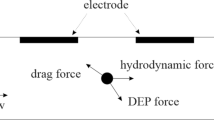
Circulating tumor cells (CTCs) detection has become one of the promising solutions for the early diagnosis of cancers. Thus, the separation of CTCs is of great importance in biomedical applications. In addition, microfluidic technology has been an attractive approach to the manipulation of biological cells. This study presents the parametric investigations relevant to the volumetric throughput of a microfluidic platform with the dielectrophoresis (DEP)-based cell manipulation technique for the continuous CTCs separation. A low potential voltage at an appropriate frequency was applied to slanted planar electrodes to separate CTCs from normal cells in blood samples due to mainly the cell size difference. The performance of the separation process was analyzed by evaluating the cell trajectories, purity, and recovery rates. Several inlet flow rates of buffer and cell sample fluid streams were examined. Various channel configurations with different outlet and height dimensions were also investigated to enhance the isolation of CTCs. During the simulation, the size and shape of cells were assumed as fixed-sized, solid spheres. The results showed that CTCs could be separated from blood cells, including white blood cells (WBCs), red blood cells (RBCs), and platelets (PLTs) with recovery and purity factors up to 100% at the cell sample throughput of 10 µL/min by utilizing a suitable microchannel design. The current study significantly contributes valuable insights into the design of the microchip devices to effectively and selectively isolate different cancerous cells in biofluids.
Graphical abstract










Similar content being viewed by others
References
Aghaamoo M, Aghilinejad A, Chen X (2017) Numerical study of insulator-based dielectrophoresis method for circulating tumor cell separation. In: Microfluidics, BioMEMS, and Medical Microsystems XV. pp 100611A–11
Alam MK, Koomson E, Zou H, Yi C, Li CW, Xu T, Yang M (2018) Recent advances in microfluidic technology for manipulation and analysis of biological cells (2007–2017). Anal Chim Acta 1044:29–65. https://doi.org/10.1016/j.aca.2018.06.054
Alazzam A, Mathew B, Alhammadi F (2017) Novel microfluidic device for the continuous separation of cancer cells using dielectrophoresis. J Sep Sci 40:1193–1200. https://doi.org/10.1002/jssc.201601061
Ali H, Park CW (2016) Numerical study on the complete blood cell sorting using particle tracing and dielectrophoresis in a microfluidic device. Korea Aust Rheol J 28:327–339. https://doi.org/10.1007/s13367-016-0033-4
Aljaghtham MS, Liu ZL, Guo JJ, He J, Celik E (2019) Numerical simulations of cell flow and trapping within microfluidic channels for stiffness based cell isolation. J Biomech 85:43–49. https://doi.org/10.1016/j.jbiomech.2019.01.010
Alshareef M, Metrakos N, Juarez Perez E, Azer F, Yang F, Yang X, Wang G (2013) Separation of tumor cells with dielectrophoresis-based microfluidic chip. Biomicrofluidics 7:1–12. https://doi.org/10.1063/1.4774312
Amstad E, Chen X, Eggersdorfer M, Cohen N, Kodger TE, Ren CL, Weitz DA (2017) Parallelization of microfluidic flow-focusing devices. Phys Rev E 95:1–6. https://doi.org/10.1103/PhysRevE.95.043105
Balakrishnan SG, Ahmad MR, Koloor SSR, Petrů M (2021) Separation of ctDNA by superparamagnetic bead particles in microfluidic platform for early cancer detection. J Adv Res. https://doi.org/10.1016/j.jare.2021.03.001
Chen J, Li J, Sun Y (2012) Microfluidic approaches for cancer cell detection, characterization, and separation. Lab Chip 12:1753–1767. https://doi.org/10.1039/c2lc21273k
Chen Y, Li P, Huang PH, Xie Y, Mai JD, Wang L, Nguyen NT, Huang TJ (2014) Rare cell isolation and analysis in microfluidics. Lab Chip 14:626–645. https://doi.org/10.1039/c3lc90136j
Choi S, Lee H, Lee S, Park I, Kim YS, Key J, Lee SY, Yang S, Lee SW (2020) A novel automatic segmentation and tracking method to measure cellular dielectrophoretic mobility from individual cell trajectories for high throughput assay. Comput Methods Programs Biomed 195:105662. https://doi.org/10.1016/j.cmpb.2020.105662
Dabighi A, Toghraie D (2020) A new microfluidic device for separating circulating tumor cells based on their physical properties by using electrophoresis and dielectrophoresis forces within an electrical field. Comput Methods Programs Biomed 185:105147. https://doi.org/10.1016/j.cmpb.2019.105147
Dalili A, Montazerian H, Sakthivel K, Tasnim N, Hoorfar M (2021) Dielectrophoretic manipulation of particles on a microfluidics platform with planar tilted electrodes. Sensors Actuators, B Chem 329:129204. https://doi.org/10.1016/j.snb.2020.129204
Dalili A, Samiei E, Hoorfar M (2019) A review of sorting, separation and isolation of cells and microbeads for biomedical applications: microfluidic approaches. Analyst 144:87–113. https://doi.org/10.1039/c8an01061g
Dalili A, Taatizadeh E, Tahmooressi H, Tasnim N, Rellstab-Sánchez PI, Shaunessy M, Najjaran H, Hoorfar M (2020) Parametric study on the geometrical parameters of a lab-on-a-chip platform with tilted planar electrodes for continuous dielectrophoretic manipulation of microparticles. Sci Rep 10:1–11. https://doi.org/10.1038/s41598-020-68699-4
Farahinia A, Zhang WJ, Badea I (2021) Novel microfluidic approaches to circulating tumor cell separation and sorting of blood cells: a review. J Sci Adv Mater Devices 6:303–320. https://doi.org/10.1016/j.jsamd.2021.03.005
Ghita M, Copot D, Ionescu CM (2021) Lung cancer dynamics using fractional order impedance modeling on a mimicked lung tumor setup. J Adv Res 32:61–71. https://doi.org/10.1016/j.jare.2020.12.016
Gupta T, Ghosh R, Ganguly R (2018) Acoustophoretic separation of infected erythrocytes from blood plasma in a microfluidic platform using biofunctionalized, matched-impedance layers. Int j Numer Method Biomed Eng. https://doi.org/10.1002/cnm.2943
Hajari M, Ebadi A, FarshchiHeydari MJ, Fathipour M, Soltani M (2020) Dielectrophoresis-based microfluidic platform to sort micro-particles in continuous flow. Microsyst Technol 26:751–763. https://doi.org/10.1007/s00542-019-04629-3
Hajba L, Guttman A (2014) Circulating tumor-cell detection and capture using microfluidic devices. TrAC - Trends Anal Chem 59:9–16. https://doi.org/10.1016/j.trac.2014.02.017
Han KH, Han SI, Frazier AB (2009) Lateral displacement as a function of particle size using a piecewise curved planar interdigitated electrode array. Lab Chip 9:2958–2964. https://doi.org/10.1039/b909753h
Hao S, Wan Y, Xia Y, Zou X, Zheng S (2018) Size-based separation methods of circulating tumor cells. Adv Drug Deliv Rev 125:3–20. https://doi.org/10.1016/j.addr.2018.01.002
Jen CP, Chen TW (2009) Selective trapping of live and dead mammalian cells using insulator-based dielectrophoresis within open-top microstructures. Biomed Microdevices 11:597–607. https://doi.org/10.1007/s10544-008-9269-1
Kazemi B, Darabi J (2018) Numerical simulation of dielectrophoretic particle separation using slanted electrodes. Phys Fluids 30:102003. https://doi.org/10.1063/1.5047153
Kenig EY, Su Y, Lautenschleger A, Chasanis P, Grünewald M (2013) Micro-separation of fluid systems: a state-of-the-art review. Sep Purif Technol 120:245–264. https://doi.org/10.1016/j.seppur.2013.09.028
Kinnunen M, Kauppila A, Karmenyan A, Myllylä R (2011) Effect of the size and shape of a red blood cell on elastic light scattering properties at the single-cell level. Biomed Opt Express 2:1803. https://doi.org/10.1364/boe.2.001803
Li M, Anand RK (2017) High-throughput selective capture of single circulating tumor cells by dielectrophoresis at a wireless electrode array. J Am Chem Soc 139:8950–8959. https://doi.org/10.1021/jacs.7b03288
Li M, Li WH, Zhang J, Alici G, Wen W (2014) A review of microfabrication techniques and dielectrophoretic microdevices for particle manipulation and separation. J Phys D Appl Phys 47:063001. https://doi.org/10.1088/0022-3727/47/6/063001
Liang W, Liu J, Yang X, Zhang Q, Yang W, Zhang H, Liu L (2020) Microfluidic-based cancer cell separation using active and passive mechanisms. Microfluid Nanofluidics. https://doi.org/10.1007/s10404-020-2331-x
Lozar T, Gersak K, Cemazar M, Kuhar CG, Jesenko T (2019) The biology and clinical potential of circulating tumor cells. Radiol Oncol 53:131–147. https://doi.org/10.2478/raon-2019-0024
Marchalot J, Chateaux JF, Faivre M, Mertani HC, Ferrigno R, Deman AL (2015) Dielectrophoretic capture of low abundance cell population using thick electrodes. Biomicrofluidics 9:054104. https://doi.org/10.1063/1.4928703
Mohamed Zackria MZA, Tirth V, Yousuff CM, Shukla NK, Islam S, Irshad K, Aarif KOM (2020) Simulation Guided Microfluidic Design for Multitarget Separation Using Dielectrophoretic Principle. Biochip J 14:390–404. https://doi.org/10.1007/s13206-020-4406-x
Nguyen NV, Jen CP (2018) Impedance detection integrated with dielectrophoresis enrichment platform for lung circulating tumor cells in a micro fluidic channel. Biosens Bioelectron 121:10–18. https://doi.org/10.1016/j.bios.2018.08.059
Nguyen NV, Jen CP (2019) Selective detection of human lung adenocarcinoma cells based on the aptamer-conjugated self-assembled monolayer of gold nanoparticles. Micromachines 10:195. https://doi.org/10.3390/mi10030195
Nguyen NV, Le Manh T, Nguyen TS, Le VT, Van Hieu N (2021) Applied electric field analysis and numerical investigations of the continuous cell separation in a dielectrophoresis-based microfluidic channel. J Sci Adv Mater Devices 6:11–18. https://doi.org/10.1016/j.jsamd.2020.11.002
Nguyen NV, Yeh JH, Jen CP (2018) A handheld electronics module for dielectrophoretic impedance measurement of cancerous cells in the microchip. Biochip J 12:208–215. https://doi.org/10.1007/s13205-015-9306-8
Okano H, Konishi T, Suzuki T, Suzuki T, Ariyasu S, Aoki S, Abe R, Hayase M (2015) Enrichment of circulating tumor cells in tumor-bearing mouse blood by a deterministic lateral displacement microfluidic device. Biomed Microdevices 17:1–11. https://doi.org/10.1007/s10544-015-9964-7
Ou X, Chen P, Huang X, Li S, Liu BF (2020) Microfluidic chip electrophoresis for biochemical analysis. J Sep Sci 43:258–270. https://doi.org/10.1002/jssc.201900758
Panklang N, Techaumnat B, Wisitsoraat A (2020) Analysis of the equivalent dipole moment of red blood cell by using the boundary element method. Eng Anal Bound Elem 112:68–76. https://doi.org/10.1016/j.enganabound.2019.12.002
Panwar N, Song P, Tjin SC, Yong K-T (2018) Sheath-assisted hydrodynamic particle focusing in higher Reynolds number flows. J Micromech Microeng 28:105018
Piacentini N, Mernier G, Tornay R, Renaud P (2011) Separation of platelets from other blood cells in continuous-flow by dielectrophoresis field-flow-fractionation. Biomicrofluidics 5:034122. https://doi.org/10.1063/1.3640045
Qian C, Huang H, Chen L, Li X, Ge Z, Chen T, Yang Z, Sun L (2014) Dielectrophoresis for bioparticle manipulation. Int J Mol Sci 15:18281–18309. https://doi.org/10.3390/ijms151018281
Sharma S, Zhuang R, Long M, Pavlovic M, Kang Y, Ilyas A, Asghar W (2018) Circulating tumor cell isolation, culture, and downstream molecular analysis. Biotechnol Adv 36:1063–1078
Song H, Rosano JM, Wang Y, Garson CJ, Prabhakarpandian B, Pant K, Klarmann GJ, Perantoni A, Alvarez LM, Lai E (2015) Continuous-flow sorting of stem cells and differentiation products based on dielectrophoresis. Lab Chip 15:1320–1328. https://doi.org/10.1039/c4lc01253d
Sun L, Yang W, Cai S, Chen Y, Chu H, Yu H, Wang Y, Liu L (2020) Recent advances in microfluidic technologies for separation of biological cells. Biomed Microdevices. https://doi.org/10.1007/s10544-020-00510-7
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F (2021) Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 71:209–249. https://doi.org/10.3322/caac.21660
Turcan I, Olariu MA (2020) Dielectrophoretic manipulation of cancer cells and their electrical characterization. ACS Comb Sci 22:554–578. https://doi.org/10.1021/acscombsci.0c00109
Yin J, Deng J, Du C, Zhang W, Jiang X (2019) Microfluidics-based approaches for separation and analysis of circulating tumor cells. TrAC - Trends Anal Chem 117:84–100. https://doi.org/10.1016/j.trac.2019.07.018
Zhang X, Xu X, Ren Y, Yan Y, Wu A (2021) Numerical simulation of circulating tumor cell separation in a dielectrophoresis based Y-Y shaped microfluidic device. Sep Purif Technol 255:117343. https://doi.org/10.1016/j.seppur.2020.117343
Zhou J, Kulasinghe A, Bogseth A, O’Byrne K, Punyadeera C, Papautsky I (2019) Isolation of circulating tumor cells in non-small-cell-lung-cancer patients using a multi-flow microfluidic channel. Microsystems Nanoeng 5:8. https://doi.org/10.1038/s41378-019-0045-6
Zhu Z, Wu D, Li S, Han Y, Xiang N, Wang C, Ni Z (2021) A polymer-film inertial microfluidic sorter fabricated by jigsaw puzzle method for precise size-based cell separation. Anal Chim Acta 1143:306–314. https://doi.org/10.1016/j.aca.2020.11.001
Acknowledgements
This work was supported by The Phenikaa University Foundation for Science and Technology Development. The authors would also like to thank Vietnam National University, Hanoi for supporting some computational tools.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have declared no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Nguyen, NV., Van Manh, H. & Van Hieu, N. Numerical simulation-based performance improvement of the separation of circulating tumor cells from bloodstream in a microfluidic platform by dielectrophoresis. Korea-Aust. Rheol. J. 34, 335–347 (2022). https://doi.org/10.1007/s13367-022-00039-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13367-022-00039-6