Abstract
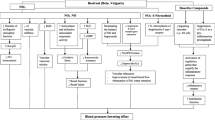
Blood is a rheologically complex suspension, in which the soluble fraction contains proteins, total cholesterol and triglycerides. The blood rheological behavior is strongly affected by the concentration of these components. This work evaluates the total cholesterol effect on the rheological behavior of Wistar rat blood by means of an in vitro study. Twenty-one rats were divided into 3 groups, each one had an assigned diet with different fat content: reference group (RG) with 3%, medium fat content group (MG) with 4.5% and high-fat content group (HG) with 6.5%; in the latter group, mixed-vegetable fat was added. From each group, intraocular representative blood samples were taken with time lapse of 15 days between each sampling followed by biochemical and hemo-rheological tests. The first analysis detected changes in total cholesterol levels, attributed to the rat metabolism, formation of adipose tissue and competition for food. The second test consisted in steady simple-shear and linear oscillatory flow. The linear viscoelastic spectra reveal that the viscous modulus is larger than the elastic modulus (Gʺ > G′), with simple-shear viscosity exhibiting shear-thinning behavior. An important finding is a pseudo-solid-like behavior at low frequencies (1 rad/s) akin to the presence of yield stresses in the high-fat content group after 30 days, revealing strong interactions between total cholesterol levels and blood cells. The hemo-rheological tests represent a promising alternative to identify pathologies present in the blood (total cholesterol, digestive problems, and diabetes).






Similar content being viewed by others
Abbreviations
- \(\% \gamma\) :
-
Deformation percentage
- \({\beta }_{i}\) :
-
ith Structural constant (BMP model)
- \(\dot{\gamma }\) :
-
Shear rate
- \({\eta }_{0,C}\) :
-
Low shear rate viscosity of isolated erythrocytes (mHAWB model)
- \({\eta }_{0}\) :
-
Zero shear rate viscosity (BMP model)
- \({\eta }_{0i}\) :
-
ith Zero shear rate viscosity (BMP model)
- \({\eta }_{\infty ,C}\) :
-
High shear rate viscosity of isolated erythrocytes (mHAWB model)
- \({\eta }_{\infty }\) :
-
High shear rate viscosity (BMP model)
- \({\eta }_{\infty i}\) :
-
ith High shear rate viscosity (BMP model)
- \({\eta }_{R}\) :
-
Structural viscosity of Rouleaux
- \({\lambda }_{C}\) :
-
Critic relaxation time for the shear-thinning behavior (mHAWB model)
- \({\lambda }_{b}\) :
-
Breakup time of the Rouleaux structures (mHAWB model)
- \({\sigma }_{y}\) :
-
Yield stress
- BMP:
-
Bautista–Manero–Puig
- EDTA:
-
Ethylenediaminetetraacetic acid
- G′:
-
Elastic modulus
- Gʺ:
-
Viscous modulus
- G 0 :
-
Shear modulus (multimodal Maxwell model)
- G 0 i :
-
ith Shear modulus (multimodal Maxwell model)
- mHAWB:
-
Modified Horner–Armstrong–Wagner–Beris
- HDL:
-
High-density lipoproteins
- HG:
-
High fat content rat group
- LDL:
-
Low-density lipoproteins
- MG:
-
Medium fat content rat group
- RG:
-
Reference rat group
- SAOS:
-
Small-amplitude oscillatory shear
- λ 0 :
-
Relaxation time (multimodal Maxwell model)
- λ 0 i :
-
ith Relaxation time (multimodal Maxwell model)
- \(K\) :
-
Power law consistency index
- \(k\) :
-
Structure-destruction constant (BMP model)
- \(n\) :
-
Power law flow index
- \(\beta\) :
-
Structural constant (BMP model)
- \(\lambda\) :
-
Dimensionless structural kinetic constant (mHAWB model)
- \(\tau\) :
-
Shear stress
- \(\omega\) :
-
Angular frequency
References
Apostolidis AJ, Beris A (2015) The effect of cholesterol and triglycerides on the steady state shear rheology of blood. Rheol Acta 55:497–509
Brooks DE (1988) Mechanism of red cell aggregation. Springer, Berlin
Buettner R, Schölmerich J, Bollheimer LC (2012) High-fat diets: modeling the metabolic disorders of human obesity in rodents. Obes Soc 15:798–808
Brust M, Aouane O, Thiébaud M, Flormann D, Verdier C, Kaestner L, Laschke MW, Selmi H, Benyoussef A, Podgorski T (2014) The plasma protein fibrinogen stabilizes clusters of red blood cells in microcapillary flows. Sci Rep 4:4348
Caballero BP, Finglas F, Toldra F (2003) Encyclopedia of food sciences and nutrition. Elsevier, Maryland
Calderas F, Herrera-Valencia EE, Sánchez-Solís A, Manero O, Medina-Torres L, Renteria A, Sánchez-Olivares G (2013) On the yield stress of complex materials. Korea-Aust Rheol J 25:233–242
Caram Y, Bautista F, Puig JE, Manero O (2005) On the rheological modelling of associative polymers. Rheol Acta 46(1):45–47
Cook RP (1958) Cholesterol: chemistry, biochemistry, and pathology. Elsevier, Scotland
Cooper RA (1978) Influence of increased membrane cholesterol on membrane fluidity and cell function in human red blood cells. J Supramol Struct 8:413–430
Cossio-Bolaños M, Gómez-Campos R, Vargas-Vitoria R, Hochmuller FR, De Arruda M (2013) Curvas de referencia para valorar el crecimiento físico de ratas machos Wistar. Nutr Hosp 28(6):2151–2156
Damiano ER (1998) The effect of the endothelial-cell glycocalyx on the motion of red blood cells through capillaries. Microvasc Res 55:77–91
Devlin TM (2010) Textbook of Biochemistry with Clinical Correlations. Wiley, New Jersey
Gelfand RA, Hendler RG, Sherwin RS (1979) Dietary carbohydrate and metabolism of ingested protein. Lancet 313:65–68
Gentsch C, Lichtsteiner M, Feer H (1988) Competition for sucrose-pellets in triads of male Wistar rats: the individuals’ performance are differing but stable. Behav Brain Res 27(1):37–44
Herrera-Valencia EE, Calderas F, Medina-Torres L, Pérez-Camacho M, Moreno L, Manero O (2017) On the pulsating flow behavior of a biological fluid: human blood. Rheol Acta 56:387–407
Högman CF, Meryman HT (1999) Storage parameters affecting red blood cell survival and function after transfusion. Transfus Med 13:275–295
Horner JS, Armstrong MJ, Wagner NJ, Beris AN (2019) Measurements of human blood viscoelasticity and thixotropy under steady and transient shear and constitutive modeling thereof. J Rheol 63:799–813
Kaibara M (1996) Rheology of blood coagulation. Biorheology 33(2):107–117
Leonhardt H, Arntz HR, Klemens UH (1977) Studies of plasma viscosity in primary hyperlipoproteinaemia. Atherosclerosis 28(1):29–40
Lowe GDO (1986) Blood rheology in arterial disease. Clin Sci 71:137–146
Macosko CW (1994) Rheology: principles, measurements and applications. Wiley-VCH, Weinheim
Maldonado-Saavedra O, Ramírez-Sánchez I, García-Sánchez JR, Ceballos-Reyes GM, Méndez-Bolaina E (2012) Colesterol: función biológica e implicaciones médicas. Revista Mexicana de Ciencias Farmaceúticas 43:7–22
Maldonado-Villamizar J (2016) Experimentación con biomodelos animales en ciencias de la salud. Avances en Biomedicina 5(3):173–177
Manero O, Pérez-López JH, Escalante JI, Puig JE, Baustista F (2007) A thermodynamic approach to rheology of complex fluids: the generalized BMP model. J Non-Newton Fluid Mech 146:22–29
Moreno L, Calderas F, Sánchez-Olivares G, Medina-Torres L, Sánchez-Solis A, Manero O (2015) Effect of cholesterol and triglycerides levels on the rheological behavior of human blood. Korea-Austr Rheol J 27:1–10
Núñez Ramírez DM, Medina Torres L, Calderas F, Lara R, Medrano Roldán H (2018) Bioleaching processfor silver recovery: Structural and rheological studies. Miner Eng 121:122–128
Núñez-Ramírez DM, Ramírez-Torres LA, Medina-Torres L, Calderas F, González Lozano MA, Ponce Peña P, Fierros Romero G, Manero O (2019) A rheological study of the bioleching process of an iron ore for the elimination of gangue minerals. Miner Eng 144:1–10
Ortiz-León G, Anaya-Luna D, Vilchez-Monge M (2014) Revisión de modelos teóricos de la dinámica de fluidos asociada al flujo de la sangre. Tecnología en marcha 27(1):66–76
Owens D, Tomkin GH (2012) LDL as a Cause of Atherosclerosis. Open Atheroscler Thromb J 5:13–21
Paredes JL, Moreno EA, Premoli G, Alarcón M, Lugo de Yarbuh A, Villareal J, Sonia A, Borges R (2009) Efectos de la ingestión de una dieta con alto contenido en grasas en ratas Wistar crónicamente infectadas con Trypanosoma cruzi. Kasmera 37(1):74–89
Reitsma S, Slaaf DW, Vink H, van Zandvoort MA, oude Egbrink MG (2007) The endothelial glycocalyx: composition, functions, and visualization. Pflugers Arch 454(3):345–359
Röhrl C, Stangl H, Wochenschr WM (2018) Cholesterol metabolism-physiological regulation and pathophysiological deregulation by the enplasmic reticulum. Wien Med Wochenschr 168:280–285
Rosenson RS, Lowe GDO (1998) Effects of lipids and lipoproteins on thrombosis and rheology. Atherosclerosis 140(2):271–280
Sarabia Aldana CA, Medina-Torres L, Calderas F, Ramírez-Torres LA, Núñez-Ramírez DM, Herrera-Valencia EE, Bernad-Bernad MJ, Manero O (2022) Hemorheological and biochemical study in patients with liver cirrhosis. Phys Fluids. https://doi.org/10.1063/5.0086561
Sousa PC, Pinho FT, Alves MA, Oliveira MSN (2016) A review of hemorheology: Measuring techniques and recent advances. Korea-Austr Rheol J 28(1):1–22
Thurston GB (1972) Viscoelasticity of human blood. Biophys J 12:1205–1217
Thurston GB (1975) Elastic effects in pulsatile blood flow. Microvasc Res 9:145–157
Tran-Son-Tay R, Sutera SP, Rao PR (1984) Determination of red blood cell membrane viscosity from rheoscopic observations of tank- treading motion. Biophys J 46(1):65–72
Varchanis S, Dimakopoulos Y, Wagner C, Tsamopoulos J (2018) How viscoelastic is human blood plasma. Soft Matter 21:1–26
Vial G, Dubouchaud H, Couturier K, Cottet-Rousselle C, Teleux N, Athias A, Galinier A, Casteilla L, Leverve XM (2011) Effects of a high-fat diet on energy metabolism and ROS production in rat liver. J Hepatol 54(2):348–356
Von Diemen V, Trindade EN, Trindale MR (2006) Experimental model to induce obesity in rats. Acta Cirurgica Brasileira 21(6):425–429
Wood D, De Bracker G, Faergeman I, Graham I, Mancia G, Pyörälä K (1998) Prevention od coronary heart disease in clinical practice: recommendations of the second joint task force of European and other societies on coronary prevention. Atheroscler J 140:199–270
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Medina-Torres, L., Calderas, F., Ramírez-Torres, L.A. et al. Rheological behavior of blood in Wistar rats with different total cholesterol levels. Korea-Aust. Rheol. J. 34, 349–358 (2022). https://doi.org/10.1007/s13367-022-00040-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13367-022-00040-z