Abstract
A study has been conducted to measure uniform corrosion due to the tunnel environment in the mass rapid transit North East Line (NEL) in Singapore. The study was aimed at investigating the corrosivity of the atmospheric environment in the NEL to enhance understanding on the maintenance of metallic components in a tunnel. The corrosivity levels at the buffer areas of ten stations along the NEL were monitored over a period of two years. The measurements were based on physical metal coupons as well as real-time monitoring systems using electrical resistance sensors. The corrosivity levels measured at different exposure sites showed differences, but were generally low and could be generally categorised as G1 according to ISA standard 71.04:2013. The reason for the low corrosivity levels was likely to be due to the relatively mild temperature and low (<60%) average relative humidity.
Similar content being viewed by others
1 Background
Corrosion refers to the degradation of a material as a result of its chemical or electrochemical reaction with the environment. For corrosion of metals to take place, the metal must be in contact with an electrolyte. The electrolyte may come from different sources, such as through immersion of a solution containing salts or from the pollutants and moisture in the atmosphere.
The most common type of corrosion is uniform (or general) corrosion, which refers to corrosion that occurs uniformly across a surface area. Uniform corrosion often results from atmospheric corrosion [1], which in turn is defined as corrosion that results from the corrosive agents present in the surrounding atmospheric environment [2–14]. A large number of studies has been conducted on atmospheric corrosion of materials including different types of steels such as carbon steels [2, 3, 5, 6] and special steels used for specific components that are exposed to the atmospheric environment [4, 7]. These studies not only aimed to elicit an understanding of the effect of the atmosphere on the corrosion of the metals, but also to develop corrosion mitigation methods such as the application of coatings [11], corrosion measurement methods [6, 12] as well as accelerated tests or prediction tools [8, 13]. Other types of corrosion refer to localised phenomena, such as pitting, crevice, galvanic or stress corrosion cracking.
Due to the prevalence of atmospheric corrosion, different standards have been developed to categorise the corrosivity of the atmospheric environment. The ISO 9223 standard [15] identifies six categories of corrosivity for outdoor environment ranging from C1 to C5 followed by the highest corrosivity category of CX. The ANSI/ISA Standard 71.04-2013 [16] identifies four corrosivity levels specifically for the electronics industry, with G1 being the lowest severity level where corrosion is unlikely to be a factor in determining equipment reliability.
The most common type of corrosion indicator consists of physical metal coupons. The mass loss of these coupons after exposure to the atmospheric environment provides an indication of the corrosivity of the environment. The measurements after 1 year of exposure may be used to extrapolate to longer duration [17]. In addition, the corrosion products on the coupons may be studied using analytical methods or microscopy such as scanning electron microscopy (SEM). For example, the morphology of corrosion products of steel may be described as sandy crystals, flowery structures, cotton balls and cigar-shaped crystal [2, 5, 18].
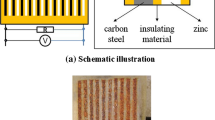
A special class of metal coupons, called environmental reactivity coupon (ERC) or corrosion classification coupon (CCC), consists of copper and silver thin strips. These coupons are used to determine the environment corrosivity levels established by ANSI/ISA-71.04-2013. The corrosion rate of such coupons is determined by an electrolytic (or cathodic) reduction technique to reveal the various types and thicknesses of corrosion films present on each coupon as well as the total amount of corrosion product [19].
For the purpose of real-time monitoring of the rate of corrosion, a number of electrochemical sensors have been developed [see recent reviews 14, 20, 21]. One sensor that has been used since 1950s is called the electrical resistance test method [14]. The electrical resistance method operates on the principle that the electrical resistance of the measuring metal element increases as its cross-sectional area decreases as the metal is corroded [22, 23]. The extent of corrosion is thereby derived by the change in resistance with respect to the initial resistance value of the sensor. Since the initial size of the sensor is known, the extent of corrosion may be calibrated against the percentage or angstroms of metal loss. The sensor end-of-life occurs when the corrosion consumes the metal element. In practice, the resistance ratio between the measuring element exposed to corrosion and the resistance of a similar reference element protected from corrosion is measured to compensate for resistivity changes due to temperature [22]. The metal element can be made of any metals—for copper and silver sensors, the measurements can be correlated with the corrosive severity levels according to ANSI/ISA-71.04-2013 similar to the ERC or CCC.
Despite the vast attention given to general atmospheric corrosion, there is a lack of studies pertaining to the corrosive atmospheric environment in railway tunnels. Most studies on corrosion of rail infrastructure have focused either on the railway track [e.g. 24–26] or on rolling stock components such as the axle and the wheel [27]. Ritter et al. [24] performed a detailed study on the corrosion fatigues of rail base, utilising methods such as microstructural analysis, salt fog testing and mechanical fatigue testing. The purpose of their tests was to compare the corrosion resistance of different coatings on the rail base. Panda et al. [26] investigated the corrosion behavior of different rail steels, while Liu et al. [28] compared the performance of different coating materials on the corrosion of overhead catenary components. In a survey performed in North America [29], various operators identified corrosion-related problems on the rail base. However, detailed studies of the corrosion incidents are not available in the public literature. Grady [30] indicated that the corrosivity level of Crossrail tunnels may be classified as C4 in areas with high seepage; however, no actual measurements had been made.
On the other hand, some research on atmospheric corrosion in road tunnel environments has been performed. Metal coupons and electrical sensors deployed to monitor corrosion in road tunnel environment indicated that the severity of corrosion varied with different months [31, 32]. Ameur-Boudabbous et al. [33] suggested that atmospheric particles and relative humidity are important factors in the process of material degradation in a road tunnel.
Due to the incidence of events relating to corrosion, and given the lack of information about the atmospheric environment and corrosion in railway tunnels, this project was initiated with a focus on developing an understanding of the corrosivity of the tunnel environment, as well as methods and tools to facilitate remote, continuous monitoring of corrosion and accelerated corrosion tests. This paper presents the results of corrosion monitoring in the North East Line (NEL) in Singapore over a period of 2 years.
2 Methodology
2.1 Field Measurements
The field measurements were performed at ten exposure sites along the NEL as tabulated in Table 1. The exposure sites consisted of specific locations at the buffer areas of ten stations that spanned the 19.2 km length of the NEL from one terminal station, Harbourfront, to the other terminal station, Punggol (see Fig. 1). The selection of the buffer area was based on the availability of space to deploy the test rack and power sockets to operate the sensors. The exposure sites were numbered #01 to #10.
2.2 Corrosion Indicators
At each exposure site, a custom-made sensor rack was installed, as shown in Fig. 2. Each rack was in the form of a floor-standing, custom-made aluminium frame which was secured to the wall. The overall size of the sensor rack was approximately 100 cm long × 30 cm wide × 20 cm deep.
Each rack allowed for the deployment of three different corrosion indicators to measure the corrosion rate, as well as an extension for a wet candle set-up. Commercially available Environmental Condition Monitoring (ECM) systems (Cosasco) were attached to the top section of the rack, CCCs were secured at the middle section and physical metal coupons were secured at the bottom section as shown in Fig. 3. The ECM sensors were installed vertically facing the track, while the CCCs were installed facing downwards. The metal coupons were installed with one side facing the direction of the moving trains.
The ECM systems were able to measure humidity, temperature and rate of corrosion on their replaceable silver and copper sensors. The corrosion rates were measured based on changes in the electrical resistance of the copper and silver sensors as corrosion took place. The measurements are expressed as metal loss in angstrom (Å), and data were logged hourly using an internal data logger. The systems required a power supply (24 V DC, 0.4 A) for continuous monitoring and an RS232 cable for data assessment.
The CCCs used in this project were supplied by Purafil (USA). Each CCC consisted of one copper and one silver strip coupon, with both coupons having been pre-mounted on an acrylic plate measuring 10.8 cm × 10.8 cm × 0.3 cm. After exposure to a predetermined period, the CCCs were sent to the vendor, Purafil, for electrolytic reduction analysis to determine the type and thickness (in Å) of the corrosion films which had built up on the coupons. The standard exposure period for a CCC is 1 month, although the ANSI/ISA-71.04-2013 standard allows shortened (for harsh environments) or extended (for mild sites) exposure times. Due to their short usage durations, the use of these coupons was discontinued in the project after half a year of usage; hence their data are not reported.
Metal coupons of three materials, AISI/SAE 4140 steel, stainless steel SS304 and copper alloy 110ETP (CDA110), were used in this study. The metal coupons were supplied by ALSPI, and their chemical composition is shown in Table 2. The coupons were in the form of rectangular plates measuring 7.62 cm × 1.27 cm × 0.15 cm. Each coupon as received was stamped with a unique serial number. Their mass and dimensions were measured and recorded before installation and after they were exposed. Photo-macrographs of the coupons before and after exposure were also recorded.
Fourteen coupons of each material were installed in the sensor rack. Two coupons of each material at each site were collected for analysis after predetermined durations. One coupon was subjected to mass loss measurements, determined according to the method specified in ASTM G1-03 (2011). The other coupon was subjected to SEM/energy-dispersive X-ray (EDX) analysis.
2.3 Exposure Period
Table 3 shows the exposure duration of the ECM systems and the metal coupons. The ECM systems were installed in May 2017, while the metal coupons were exposed from June 2017 onwards. For this manuscript, data between June 17 and May 19 corresponding to 24 months of exposure are reported for the ECM measurements and the 4140 steel coupons. For the copper and stainless steel coupons, the results corresponding to 12 months of exposure are reported.
2.4 Metal Loss Measurement
The metal loss due to corrosion was measured according to the ASTM G1-03 and ISO 9226 procedures. Where applicable, the corrosion product on the surface of the coupons was carefully removed by immersing the coupons in the relevant pickling solutions (Table 4) for 1 min, followed by sonication in ethanol for another minute and then air drying. The coupons were then weighed for the mass loss due to corrosion. To ensure that the corrosion product was removed completely, this process was repeated until the weight of the samples reached a constant value (within a tolerance of ±0.5 mg).
2.5 Microstructural Analysis
The morphology of the corrosion products was studied using a scanning electron microscope (model JSM-IT300LV, JEOL) at an accelerating voltage of 10 kV. EDX spectroscopy was carried out to detect elements in the corrosion product. Where applicable, the corrosion products were also subjected to X-ray diffraction (XRD) analysis using a Bruker D2 PHASER diffractometer. The XRD analysis was based on copper anode radiation with a scanning rate of 0.04°/min and with 2θ from 10° to 90°. Powder diffraction files (PDF) were used for the XRD semi-quantitative analysis.
3 Results and Discussion
3.1 Temperature and Relative Humidity
The data for temperature and relative humidity at the exposure sites are shown in Figs. 4 and 5, respectively. The measurements were made using the ECM systems over a duration of 24 months, with a data capture rate of one data point per hour. The recorded temperatures were largely between 30 and 35 °C, while the recorded relative humidity values were largely between 40% and 60%. Compared with the outside atmosphere, where the mean monthly relative humidity values were typically above 70%Footnote 1, the values of the relative humidity at the buffer areas were significantly lower. Between the different exposure sites, relatively small differences can be observed.
The temperature and relative humidity data are replotted as box-plots in Figs. 6 and 7, respectively. Minor fluctuations can be observed during the day when the trains were operating, but larger fluctuations tended to occur during the hours of non-operation (between 1 am and 5 am). As a result of the fluctuations, the relative humidity at some exposure sites reached values above 70%. These relatively large fluctuations could be due to the operations of the tunnel ventilation fan during maintenance works, which would draw the outside atmosphere into the buffer areas.
3.2 Corrosion Rate Measurement from Environmental Condition Monitoring (ECM) Systems
The metal losses of the silver and copper sensors of the ECM are shown in Figs. 8 and 9, respectively. The silver sensors at all exposure sites showed measurable metal loss. Site #01 recorded the highest metal loss, and its average corrosion rate was approximately twice the average corrosion rate at site #4, which recorded the lowest metal loss. For the copper sensor, almost no corrosion was detected over the 2-year duration. Despite the variations among the different sites, the metal loss from all ten exposure sites corresponded to the G1 category according to the ANSI/ISA-71.04-2013.
3.3 Corrosion Rate Measurements from Physical Coupons
The appearance of the metal coupons after 12 months of exposure is shown in Figs. 10, 11 and 12. Corrosion can be clearly observed on the 4140 steel coupons, but apart from traces of a small amount of stain, very little corrosion is observed on the copper and stainless steel coupons.
The mass loss of the 4140 steel coupons is shown in Fig. 13. For site #01, the corrosion rate within the first year was high, but this was followed by a plateau. For sites #04, #06 and #08, a gradual decrease in the corrosion rate can be observed. Sites such as #02, #07, #09 and #10, show an irregular increase in corrosion during the exposure duration.
The maximum thickness reduction was detected for site #01 after 12 months and site #10 after 24 months of exposure. After 24 months of exposure, the largest thickness reduction was slightly higher than 2 µm and about two to three times larger than the smallest thickness reduction corresponding to site #07. For low carbon steels, ISO 9223:2012 indicates that the ranges of thickness reduction due to corrosion for the lowest two categories, C1 and C2, are 0–1.3 μm/year and 1.3–25 μm/year, respectively. Since the steel used in this study was 4140 with 0.38 wt% carbon, the ISO 9223:2012 corrosion categories are not directly applicable. Nonetheless, the corrosion rates exhibited by the 4140 steel coupons appear to compare to the lower range of the corrosion rates of the low carbon steels. For copper, the amount of thickness reduction after 1 year of exposure is summarised in Table 5. The values at all exposure sites were less than 0.1 µm, which can be categorised as C1 in accordance with ISO 9223:2012.
ISO 9223 (2012) uses a time-of-wetness (TOW) parameter to determine the presence of a thin layer of electrolyte on metal surfaces to effect a high corrosion rate. The TOW parameter is defined as the time when temperature is above 0°C and relative humidity is above 80%. At the present exposure sites, the relative humidity was found to be mostly below 80%; hence the TOW parameter is not applicable. Other studies have suggested that a thin film of moisture may form on the surface at about 60% relative humidity [34, 35]. The number of data points corresponding to a relative humidity above 60% was therefore determined at each site, and correlated with both the silver corrosion from the ECM systems and the mass loss from the 4140 metal coupons in Figs. 14 and 15, respectively. The data suggest a possible correlation between the silver corrosion and sites with a higher frequency of relative humidity >60%. The data for the 4140 metal coupons, however, show a larger scatter and weaker correlation. For copper, both the copper sensor in the ECM systems and the copper metal coupons show little corrosion. The low degree of copper corrosion is likely due to the comparatively low relative humidity at the exposure sites, as previous work [36] suggests that copper corrosion takes place above a critical relative humidity of about 63–75%. The slightly higher degree of corrosion of the copper sensor detected for sites #01 and #06 in Fig. 9 may be correlated with the higher frequency of relative humidity >60% at these sites.
Figure 16 shows the correlation between the measured increase in mass of the 4140 steel coupons as a result of the corrosion and their corresponding mass loss after the corrosion products were removed. The data suggest a direct relationship between the mass gain and the mass loss, such that a quick estimate of the extent of corrosion of the coupons may be obtained by measuring the mass gained by the coupons if access to more sophisticated laboratory facilities is not possible. Since the mass gain may be influenced by the composition of the corrosion products and absorbed moisture as well as other surface effects [37, 38], the measurement of mass loss should be performed for more precise evaluation.
3.4 Morphology of the Corrosion Products
Figure 17 shows the representative morphology of the corrosion products of 4140 steel coupons. It can be observed that the morphology consisted largely of nodular structures with flowery needle-like rust. The XRD analysis of the corrosion products, as presented in Fig. 18, suggests that the corrosion products consisted of iron oxides and iron oxhydroxides, such as lepidocrocite, goethite, magnetite and akaganeite. These morphological features and corrosion products are similar to those found on steels exposed to atmospheric corrosion as reported by other studies [2, 39, 40]. SEM/EDX analyses of the exposed 4140 steel coupons revealed the presence of sulfur and chlorine elements, which are likely the main corrosive agents in the tunnel. Sulfur and chlorine are common corrosive elements from the atmosphere, and previous studies on atmospheric corrosion of steels have detected the same elements [3, 39].
4 Conclusions
The ‘profile’ of the macro-climate of the NEL tunnel atmospheric environment, in terms of the relative humidity and temperature, has been determined. Although some differences in the corrosivity levels were measured at different exposure sites, the order of magnitude of the uniform corrosion was overall low. The low level of corrosivity is likely due to the relatively low average relative humidity values, which were below 60%. These results contributed to the understanding of the tunnel corrosivity profile and would be useful in developing appropriate maintenance programmes for the metallic components in a tunnel. A limitation of the study was that the exposure sites were limited to the buffer areas and similar measurements were not made away from the buffer areas. Electrochemical methods, in the form of an electrical resistance method, have potential for real-time monitoring of the corrosion rate in the tunnel. Further studies could be conducted to improve on these methods.
Notes
Data can be found at https://data.gov.sg/dataset/relative-humidity-monthly-mean
References
Davis J (2000) Corrosion: understanding the basics. American Technical Publishers Ltd, Materials Park, Ohio
Alcantra J, Chico B, Diaz I, Fuente DDL et al (2015) Airborne chloride deposit and its effect on marine atmospheric corrosion of mild steel. Corros Sci 97:74–88
Fuente DDL, Diaz I, Simancas J et al (2011) Long-term atmospheric corrosion of mild steel. Corros Sci 53:604–617
Feng C, Xie Y et al (2018) The corrosion behavior of T/P91 steel under the atmosphere environment in Hunan province. MATEC Web Conf 175:01002
Xiao K, Cf D, Li XG et al (2008) Corrosion products and formation mechanism during initial stage of atmospheric corrosion of carbon steel. J Iron Steel Res 15:42–48
Ma XZ, Meng LD, Cao XK et al (2022) Investigation on the initial atmospheric corrosion of mild steel in a simulated environment of industrial coastland by thin electrical resistance and electrochemical sensors. Corros Sci 204:110389
Díaz I, Cano H, Lopesino P et al (2018) Five-year atmospheric corrosion of Cu, Cr and Ni weathering steels in a wide range of environments. Corros Sci 141:146–157
Song X, Wang K, Zhou L et al (2022) Multi-factor mining and corrosion rate prediction model construction of carbon steel under dynamic atmospheric corrosion environment. Eng Fail Anal 134:105987
Grøntoft T (2021) Atmospheric corrosion due to amine emissions from carbon capture plants. Int J Greenh Gas Con 109:103355
Pei Z, Cheng X, Yang X et al (2021) Understanding environmental impacts on initial atmospheric corrosion based on corrosion monitoring sensors. J Mater Sci Technol 64:214–221
Lazorenko G, Kasprzhitskii A, Nazdracheva T (2021) Anti-corrosion coatings for protection of steel railway structures exposed to atmospheric environments: a review. Constr Build Mater 288:123115
Shan WAN, Liao BK, Dong ZH, Guo XP (2021) Comparative investigation on copper atmospheric corrosion by electrochemical impedance and electrical resistance sensors. Trans Nonferrous Met Soc China 31(10):3024–3038
Jin YH, Ha MG, Jeon SH et al (2020) Evaluation of corrosion conditions for the steel box members by corrosion monitoring exposure test. Constr Build Mater 258:120195
Popova K, Prošek T (2022) Corrosion monitoring in atmospheric conditions: a review. Metals 12:171
ISO 9223 (2012) Corrosion of metals and alloys—Corrosivity of atmospheres—Classification, determination and estimation.
ISA-71-04 (2013) Environmental conditions for process measurement and control systems: Airborne contaminations
ISO 9226 (2012) Corrosion of metals and alloys—corrosivity of atmospheres—Determination of corrosion rate of standard specimens for the evaluation of corrosivity
Antunes RA, Costa I, Faria DLAD et al (2003) Characterization of corrosion products formed on steels in the first months of atmospheric exposure. Mater Res 6:403–408
Muller CO (1990) Combination corrosion coupon testing needed for today’s control equipment. Pulp Paper Magaz 64:165–169
Xia DH, Song S, Qin Z et al (2019) Electrochemical probes and sensors designed for time-dependent atmospheric corrosion monitoring: fundamentals, progress, and challenges. J Electrochem Soc 167(3):037513
Xia DH, Deng C, Macdonald D et al (2022) Electrochemical measurements used for assessment of corrosion and protection of metallic materials in the field: a critical review. J Mater Sci Technol 112:151–183
ASTM G96-90 (2018) Standard guide for online monitoring of corrosion in plant equipment (electrical and electrochemical methods)
Dubus M, Kouril M, Nguyen T et al (2010) Monitoring copper and silver corrosion in different museum environments by electrical resistance measurement. Stud Conserv 55:121–133
Ritter GW, Mohr WC, Jeong DY, et al. (2014) Rail base corrosion and cracking prevention. http://www.fra.dot.gov/Elib/Document/3959. Accessed on 01/01/2020
Maruthamuthua S, Dhandapania P, Kamalasekaran S et al (2013) Role of ureolytic bacteria on corrosion behavior of fretted grade 880 mild steel rail. Eng Fail Anal 33:315–326
Panda B, Balasubramaniam R, Dwivedi G (2008) On the corrosion behaviour of novel high carbon rail steels in simulated cyclic wet–dry salt fog conditions. Corros Sci 50:1684–1692
Beretta S, Carboni M, Fiore G et al (2010) Corrosion–fatigue of A1N railway axle steel exposed to rainwater. Int J Fatigue 32:952–961
Liu LR, Yong XY, Guo FY (2015) Research on the corrosion of metallic components of overhead contact system in typical atmospheric environment. J Rail Eng Soc 3:81
TRB (2007) Transit Cooperative Research Program (TCRP) web-only document 37: rail base corrosion detection and prevention. http://www.trb.org/Publications/Blurbs/159033.aspx. Accessed 01/01/2020
Grady (2017) Crossrail Project: Infrastructure design and construction. ICE publishing
Kreislova K, Geiplova H, Licbinsky R, et al. (2012) Corrosivity of road tunnel microclimate. EUROCORR 2012
Kreislova K, Knotkova D (2011) Corrosion behaviour of structural metals in respect to long-term changes in the atmospheric environment. EUROCORR 2011
Ameur-Boudabbous I, Kasperek J, Barbier A et al (2014) Transverse approach between tunnel environment and corrosion: particulate matter in the Grand Mare tunnel. J Air Waste Manag Asso 64:198–218
Cole IS, Ganther WD, Sinclair JD et al (2004) A study of the wetting of metal surfaces in order to understand the processes controlling atmospheric corrosion. J Electrochem Soc 151:B627–B635
Tullmin M, Roberge PR (1995) Corrosion of metallic materials. IEEE Trans Reliab 44:271–284
Vernon WHJ (1931) A laboratory study of the atmospheric corrosion of metals. Part I.—the corrosion of copper in certain synthetic atmospheres, with particular reference to the influence of sulphur dioxide in air of various relative humidities. Trans Faraday Soc 27:255–277
Strebl M, Bruns M, Virtanen S (2020) Respirometric in situ methods for real-time monitoring of corrosion rates: Part I. Atomospheric corrosion. J Electrocheml Soc 167:021510
Johansson E, Leygraf C, Rendahl B (1998) Characterisation of corrosivity in indoor atmospheres with different metals and evaluation techniques. Br Corros J 33:59–66
Castano JG, Botero CA, Restrepo AH et al (2009) Atmospheric corrosion of carbon steel in Colombia. Corros Sci 52:216–223
Balasubramanian R, Ramesh Kumar AV (2003) Corrosion resistance of the Dhar iron pillar. Corros Sci 45:2451–2465
Acknowledgements
Research reported in this publication was supported by the Land Transport Innovation Fund of the Land Transport Authority of Singapore (LTA). We would also like to acknowledge the support from LTA and SBS Transit teams for provision of data and facilitating tunnel access and site installations.
Funding
Land Transport Authority Singapore.
Author information
Authors and Affiliations
Contributions
SM Goh 40%, LT Tan 30%, HY Gan 15%, YL Foo 5%, KH Goh 5%, HS Lee 5%.
Corresponding author
Ethics declarations
Conflict of interest
None.
Additional information
Communicated by Liang Gao.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Goh, S.M., Tan, L.T., Gan, H.Y. et al. Monitoring of Atmospheric Corrosion in a Mass Rapid Transit (MRT) Tunnel. Urban Rail Transit 8, 184–197 (2022). https://doi.org/10.1007/s40864-022-00172-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40864-022-00172-z