Abstract
Introduction
COVID-19 infection develops neurologic symptoms such as smell and taste loss. We aimed to determine the volumetric changes in the brain and correlation of possible related biochemical parameters and endocannabinoid levels after COVID-19 recovery.
Methods
Brain magnetic resonance images of recovered COVID-19 patients and healthy volunteers, whose olfactory and gustatory scores were obtained through a questionnaire, were taken, and the volumes of the brain regions associated with taste and smell were measured by automatic and semiautomatic methods. Endocannabinoids (EC), which are critical in the olfactory system, and vitamin B12, zinc, iron, ferritin, thyroid-stimulating hormone (TSH), and thyroxine (T4) levels, which are reported to have possible roles in olfactory disorders, were measured in peripheral blood.
Results
Taste and smell disorder scores and EC levels were found to be higher in recovered COVID-19 patients compared to controls. EC levels were negatively correlated with bilateral entorhinal cortex (ENT) volumes in the COVID-19 group. Subgenual anterior cingulate cortex volumes showed correlations with gustatory complaints and ferritin in recovered COVID-19 patients.
Conclusions
The critical finding of our study is the high EC levels and negative correlation between EC levels and left ENT volumes in recovered COVID-19 patients.
Implications
It is possible that ECs are potential neuromodulators in many conditions leading to olfactory disorders, including COVID-19.
Similar content being viewed by others
Introduction
In February 2020, the virus that was initially called 2019-nCoV and the Wuhan coronavirus was officially named the “severe acute respiratory syndrome coronavirus 2” (SARS-CoV-2). The World Health Organization (WHO) renamed the disease caused by this virus as the novel coronavirus disease 2019 (COVID-19) (Lai et al. 2020). Although SARS-CoV-2 infection mainly causes respiratory tract problems, in some patients, neurological symptoms such as headache, loss of taste and smell, ataxia, meningitis, cognitive dysfunction, amnesia, seizures, and impaired consciousness develop (Bougakov et al. 2020; Carod-Artal 2020; Harapan and Yoo 2021). It was thought that the virus causes these neuronal symptoms by migrating to the brain (Bougakov et al. 2020; Carod-Artal 2020; Harapan and Yoo 2021). While loss of smell (anosmia) and/or loss of taste (ageusia) are now considered to be among the main symptoms of several coronavirus variants that cause COVID 19 disease (Coelho et al. 2022), there are also reports of anosmia and/or ageusia cases without any other symptom (Hajikhani et al. 2020). Two hypotheses have been proposed regarding the formation mechanisms of these symptoms. The first hypothesis is that the virus migrates to and harms the olfactory bulb (OB) or the central olfactory pathways through the olfactory neuroepithelium in the olfactory mucosa (Bougakov et al. 2020). The second hypothesis is about hematogenous spread. SARS-CoV-2 uses the human cell receptor angiotensin-converting enzyme 2 (ACE2) as its cellular receptor (Ou et al. 2020). It was argued that SARS-CoV-2 creates neuronal damage by causing endothelial ruptures in capillaries that carry the ACE2 receptor (Baig et al. 2020). In the central nervous system (CNS), ACE2 is expressed in many regions of the human brain, especially in the brain stem nuclei associated with cardiorespiratory function, the amygdala, and the cerebral cortex (Coolen et al. 2020; Lukiw et al. 2022). Ageusia may be secondary to olfactory dysfunctions, whereas it can also develop as a result of the interaction of the SARS-CoV-2 virus with the ACE2 receptor in the host. This is because the mucosal epithelial cells of the oral cavity contain the ACE2 receptor abundantly (Xu et al. 2020).
What is known about changes in cases of loss of sense of smell has increased with voxel-based morphometry (VBM) analyses (Bitter et al. 2010a). A volumetric reduction in OB in loss of smell or olfactory dysfunctions is a known phenomenon, such that the degree of the reduction in the volume of OB is correlated with the severity of loss of smell (Bitter et al. 2010b; Mueller et al. 2005). After the sensation of smell reaches OB, it is transferred to the olfactory cortex (OLC) from there (Bitter et al. 2010b; Han et al. 2019). OLC contains a few regions such as the piriform cortex (PC), entorhinal cortex (ENT), amygdala, anterior olfactory nucleus (AON), and olfactory tubercle (OT) (Bitter et al. 2010b). These regions are closely connected to the anterior insular cortex (AIC), orbitofrontal cortex (OFC), thalamus, hippocampus (HIPPO), and anterior cingulate cortex (ACC) (Bitter et al. 2010a, 2010b). Other than OB, VBM analyses have revealed volumetric losses of gray matter in secondary olfactory areas like the insular cortex, ACC, OFC, fusiform gyrus, precuneus, middle temporal gyrus (MTG), and PC (Bitter et al. 2010a).
The endocannabinoid system (ECS), which is a neuromodulator system that has a critical role in the regulation of homeostasis with its receptors that are expressed prevalently in many tissues of the body, also takes part in olfactory processes (Maccarrone et al. 2015). Studies have shown that ECS is not only effective on neurogenesis in the adult OB, but it also functionally contributes to the neuronal networks of the olfactory system (Bhatia-Dey and Heinbockel 2020). Different stimuli on OB lead to ECS activation (Bhatia-Dey and Heinbockel 2020). The levels of endocannabinoids (ECs) in circulation increase in various cases involving systemic inflammation (Hillard 2018). It is thought that ECS activation may be a potential cause of the onset and progression of olfactory dysfunctions by leading to the formation of disrupted gene regulation networks (Bhatia-Dey and Heinbockel 2020).
Additionally, it has been reported that hypothyroidism (Hummel et al. 2012; Świdziński et al. 2016), vitamin B12 deficiency (Hummel et al. 2012), and iron deficiency (Hansen et al. 2017) can lead to olfactory dysfunctions. These values are among laboratory tests that are recommended for patients with non-specific causes of their taste and smell dysfunctions.
The purpose of this study is to examine volumetric changes in the central olfactory and gustatory regions with the VBM analysis of magnetic resonance (MR) images and investigate the relationship between EC levels, vitamin B12, zinc, iron, ferritin, free thyroxine (T4), and thyroid-stimulating hormone (TSH) in peripheral blood in patients recovered from COVID-19 but still express olfactory and gustatory complaints.
Materials and Method
Patient Selection
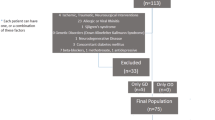
This research was approved at the meeting of Kastamonu University Clinical Research Ethics Committee on 26/11/2020 (decision no: 2020-KAEK-143–13). The COVID-19 group was created from COVID-19 patients who were diagnosed with COVID-19 with a PCR test in the Otorhinolaryngology Outpatient Clinic of the Kastamonu Research and Training Hospital. Twenty patients who recovered from COVID-19 but still express olfactory and gustatory complaints included. It had been 1 to 3 weeks since the patients got over the disease when they were included in the study. Twenty healthy individuals who did not have any smell and taste dysfunction or loss were included into control group. Individuals in the COVID-19 group and control individuals had no other disease known to affect their chemical senses. Participation in the study was on a voluntary basis, and the participants provided consent.
Smell and Taste Questionnaire
In our study, first, medical history was taken from the participants regarding changes they experienced in their senses of smell and taste, if any. For this purpose, a modified version of the validated Monell-Jefferson Taste–Smell Questionnaire (the Monell Chemical Senses Center) was created with the support of an otorhinolaryngology specialist and an expert statistician. The modified questionnaire consisted of 9 questions to get feedback on whether there is a disorder in the sense of smell and to get the details of it if there is and 8 questions to obtain the same information for the sense of taste. The questions were about the direction of the change in the sense of smell or taste (such as disappearance, decrease, increase, phantom smell), in what term the change occurred, and whether it was unilateral or bilateral. In addition, changes in identifying daily odors such as cigarette smoke, gasoline, vinegar, rose and sweet, salty, sour, and bitter tastes were asked. In addition, people were asked if they had taken any medication, surgery, supplements, or any dental treatment. Then, the information was quantitatively coded, and the scores for the degree of smell and taste loss were calculated for each participant.
Voxel-Based Morphometry and MRICloud Analysis
In order to analyze the volumetric changes in the brain regions associated with the senses of smell and taste, 3-dimensional (3D) cranial MR images of the participants were obtained. The volumes of the olfactory cortex, insula, hippocampus, amygdala, orbitofrontal cortex, angular gyrus, and thalamus were analyzed using the VBM8 toolbox working in SPM8 in MATLAB 7.12 (R2011a) (the Wellcome Trust Centre for Neuroimaging, UK). T1 images were normalized, segmented, and smoothed respectively, and standard routines and default parameters of the VBM8 toolbox were applied. For determining the volumes of the entorhinal cortex, orbitofrontal gyrus, and anterior cingulate cortex, hdr/img files were uploaded to the MRICloud website (https://braingps.mricloud.org/), and automated segmentation and volumetric analysis were performed (Figs. 1 and 2). OB volume was measured separately by an experienced neuroradiologist and an experienced neuroanatomist separately using ImageJ Segmentation Editor (Fig. 3). Each observer made the measurement twice blindly, and the mean of all measurements was recorded.
Endocannabinoid Test Procedure
Blood samples were collected in biochemistry tubes containing gel for serum separation and were centrifuged and then taken into cryovial tubes and stored at − 80 °C. In the sera obtained by centrifugation, EC levels were measured with the ELISA method based on the manufacturer’s instructions (Cayman Chemicals, Michigan, ABD).
Statistical Analysis
In this study, the collected data were analyzed in a 95% confidence interval using the IBM SPSS (Statistical Package for the Social Sciences Statistics) version 22 software. As n < 30 and the data were not normally distributed, for the volumes of smell- and taste-related brain regions, peripheral blood levels of ECs and biochemical parameters and the Smell and Taste Questionnaire scores were compared by the non-parametric Mann–Whitney U test between the groups. Spearman’s rank correlation coefficient was used for the correlation analysis. Significance levels were determined for all comparisons by applying Bonferroni correction.
Results
The Mean Comparisons Between Groups
Demographic Characteristics
This study included 12 male and 8 female patients in the COVID-19 group and 11 male and 9 female individuals in the control group. The sex distributions in the two groups were similar (p = 0.138). The mean age of the COVID-19 group was 34.25 ± 13.05, while the mean age of the control group was 32.20 ± 9.89. There was also no significant difference between the mean ages of the two groups (p = 0.375).
Volumes of Brain Regions Associated with Taste and Smell
Statistically significant differences were found between the COVID-19 and control groups in terms of the volumes of two brain regions. While the right angular gyrus volume was significantly smaller in the COVID-19 group than controls (p = 0.037), the left ENT volume was significantly higher (p = 0.046). On the other hand, the left angular gyrus and the right ENT volumes, in addition to other observed regions, did not differ between groups (Table 1).
Endocannabinoid Levels in Peripheral Blood
The mean EC level in the peripheral blood of the COVID-19 group (3.74 ± 1.58) was found to be significantly higher than the mean EC level of the control group (2.65 ± 0.30) (p = 0.005).
Biochemical Parameters in Peripheral Blood
No significant difference was found between the groups in terms of the biochemical parameters (B12, zinc, iron, ferritin, T4, TSH) examined in their peripheral blood (Table 2).
Smell and Taste Questionnaire Results
The olfactory disorder score (1.55 ± 0.89) and gustatory disorder score (1.45 ± 1.19) of the COVID-19 patients were significantly higher than control individuals’ olfactory disorder score (0.00 ± 0.00) and gustatory disorder score (0.00 ± 0.00) (p = 0.000 for both). According to the formula created, the higher the score, the higher the sensory deterioration. Sensory disorders were scored with a positive number when evaluating questionnaires. Since no one in the control group had any taste or smell disorders, their scores were found to be zero. The highest questionnaire score for the olfaction and taste was 3.
Correlation Analysis Within the Group
In the COVID-19 group, gustatory disorder score had positive, moderate correlation with left and right subcallosal ACC volume (respectively, r = 0.594, p = 0.028; and r = 0.412, p = 0.046). In the COVID-19 patients, the left subgenual ACC volume had positive, moderate correlation with ferritin level (r = 0.448, p = 0.047).
The left and right ENT volumes had negative, moderate correlation with the EC levels in COVID-19 group (respectively, r = − 0.554, p = 0.011; r = − 0.584, p = 0.007).
Discussion
Anosmia or partial loss of smell, which is usually accompanied by changes in taste, is a frequently encountered symptom that is helpful in the diagnosis of COVID-19 (Hopkins et al. 2020; Marinosci et al. 2020). While it is generally a temporary phenomenon that lasts for a few weeks, cases of long-term loss have also been reported (Aragão et al. 2020). This made us think of possible gray matter changes. Indeed, for ENT, which is named the secondary olfactory cortex, the low volumes of the left ENT in the COVID-19 group than controls in our study implicated the presence of dominant lateralization. The right ENT did not show a significant difference between the groups. Previously, Strauss et al. (2020) discovered no signal anomalies in the orbitofrontal and entorhinal cortices and other olfactory pathways, but they reported increased entorhinal and orbitofrontal cortex signal anomalies in only one COVID-19 patient (Strauss et al. 2020). We can speculate that there is a relationship between increased signaling and increased volume.
An interesting result we obtained was that the volume of the right angular gyrus was significantly smaller in recovered COVID-19 patients. Model studies have shown that sensory deprivation alters neuronal populations in multisensory areas (Carriere et al. 2007). Iravani et al. (2021) found that in individuals with acquired anosmia, the angular gyrus is activated to integrate multisensory information with its function of visual-auditory integration, and the nervous system tries to compensate for the disrupted olfactory information by multisensory integration including visual-auditory information (Iravani et al. 2021). However, in our study, the right gyrus angularis was seen to have a lower volume in the recovered COVID-19 patients than the controls. We think that the reduction in the volume of the right angular gyrus, which is a heteromodal association area, may be related to olfactory and taste dysfunctions.
Due to the potential of the virus to severely disrupt many vital organs (Wang et al. 2020), analyzing biochemical factors is a suitable. Although there is no sufficient evidence, it has been reported that deficiencies of copper, zinc, vitamin A, vitamin B6, and vitamin B12 can cause olfactory dysfunction (Derin et al. 2006; Doty et al. 2006).
Zinc not only plays an important role in the immune system where it works as a signal molecule but also helps the synthesis of the protein gustin that is associated with the formation of taste buds (Ambaldhage et al. 2014). Tomita (1990) found in his study where they administered radioactive zinc to rats with experimentally induced zinc deficiency and examined autoradiographs that silver particles mostly concentrated in taste buds (Tomita 1990). Additionally, Tomita (1990) reported that low serum zinc levels lead to gustatory dysfunctions related to zinc deficiency (Tomita 1990). Jothimani et al. (2020) obtained lower zinc levels in COVID-19 patients than healthy controls (Jothimani et al. 2020). In our study, while the mean serum zinc level of the COVID-19 patients was slightly higher than that in the control group, this difference was not statistically significant. This result suggested that many factors such as the stage of infection, viral load, and individual variations in the immune system may lead to conflicting results by affecting serum zinc levels in COVID-19 cases.
Derin et al. (2006) reported that olfactory dysfunction may originate from myelin damage in the olfactory nerve associated with vitamin B12 deficiency and the toxic effects of high homocysteine levels on the nervus olfactorius (Derin et al. 2006). They reported in their study that people with vitamin B12 deficiency showed significant dysfunctions in all smell test parameters. According to the results of our study, the olfactory disorders associated with COVID-19 may not be associated with B12 because no significant difference was observed between the groups.
Serum ferritin is an iron storage protein that is measured as a marker of iron levels, while it is also a well-known inflammatory marker (Lin et al. 2020). Pourbagheri-Sigaroodi et al. (2020) reported the mean rate of increase in the ferritin levels of COVID-19 patients after viral infection as 275% (Pourbagheri-Sigaroodi et al. 2020). Lin et al. (2020) stated that serum ferritin levels showed a correlation with the severity of systemic and pulmonary inflammation, and high serum ferritin levels could predict the risk of increased disease severity in COVID-19 patients (Lin et al. 2020). Excess intracellular iron creates reactive oxygen species (ROS) by interacting with molecular oxygen and causes ferroptosis, which is a programmed cell death process (Kell and Pretorius 2014). Ferroptosis has been found to be associated with neurological disorders including ageusia and anosmia (Dinç et al. 2016). We also thought that taste and smell disorders in COVID-19 might be related to ferritin levels, but we did not detect such a significance. Measuring ferritin levels may be meaningful during the course of the disease, and the change in ferritin levels may disappear after recovery. We do not have enough data to comment on whether the transient increase in ferritin level will lead to persistent neurological disorders. Although the ferritin levels did not show a significant difference between the two groups, the volumes of the left subgenual ACC in COVID-19 group were also positively and strongly correlated with ferritin levels.
Studies performed by Mackay-Sim and Beard (1987) on mice have revealed that thyroxine is required for the normal development of the nervous system including the growth of new olfactory receptor neurons (Mackay-Sim and Beard 1987). Świdziński et al. (2016) determined that hypothyroidism affected the sense of smell significantly, weakened it, and even suppressed it completely (Świdziński et al. 2016). Baskoy et al. (2016) observed higher TSH and T3 levels and lower T4 levels in hypothyroidism patients who had olfactory and gustatory dysfunctions in comparison to the control group (Baskoy et al. 2016). In our study, while the TSH values were minimally higher, T4 values were lower in the COVID-19 patients than the healthy controls; these differences were not statistically significant. In this case, it can be thought that thyroid hormones do not play a role in COVID-19-related olfactory disorders.
The most interesting result of our study contains the ECS. ECs affect the sense of smell directly or indirectly (Czesnik et al. 2007). They show their endogenous effects through CB1 and CB2 receptors (Wang et al. 2012). In our study, the mean EC level values in the peripheral blood samples of the COVID-19 group were found to be significantly higher than those in the control group. The EC levels were negatively and moderately correlated with the left ENT volume. Consistent with our results, previously cannabis usage was reported to be related with reduced ENT thickness (Levar et al. 2018). This result is not surprising since one of the areas where CB1 receptors diffuse throughout the central nervous system is the ENT (Sinclair 2016). However, the lack of such a relationship in other areas where EC receptors are located indicates that factors other than ECs may also be effective here. In a recent study, it was found that EC levels were also increased in Alzheimer’s patients (Petekkaya et al. 2022). Recent studies have stated that there are Alzheimer-like changes in the brains of COVID-19 patients (Reiken et al. 2022) and that Alzheimer’s and COVID-19 have common genetic risk factors (Magusali et al. 2021). Based on these results, we think that the role of ECs in the symptoms of COVID-19 can be further investigated.
Conclusions
In this study, the volumetric changes of pathways and primary brain regions associated with smell/taste and changes in smell/taste identification capacities in recovered COVID-19 patients and healthy controls, as well as the relationships of these changes to some biochemical parameters and serum EC levels, were investigated. Our results demonstrated that short-term acquired anosmia is related to altered GM volumes in both cortical regions associated with olfactory functions and those associated with multisensory integration, ECs that have a neuromodulation role step in at the onset of olfactory dysfunction, and they may increase the dynamic functional connections between these regions. More research is necessary to understand whether or not GM volume alterations occurring in cortical regions in COVID-19 cases could be the main reason for olfactory dysfunctions that can be permanent after COVID-19 infection.
Limitations
Although this study showed changes in the brains of recovered COVID-19 patients in the olfactory-related areas and in the multimodal association areas and the possible neuromodulatory role of ECS, the fact that smell and taste disorders were evaluated only with the questionnaire method is an important limitation for the study.
References
Ambaldhage VK, Puttabudd JH, Nunsavath PN, Tummuru YR (2014) Taste disorders: a review. J Indian Acad Oral Med Radiol 26(1):69. https://doi.org/10.4103/0972-1363.141864
Aragão MFVV, Lea MC, Cartaxo Filho OQ, Fonseca TM, Valença MM (2020) Anosmia in COVID-19 associated with injury to the olfactory bulbs evident on MRI. Am J Neuroradiol 41(9):1703–1706. https://doi.org/10.3174/ajnr.A6675
Baig AM, Khaleeq A, Ali U, Syeda H (2020) Evidence of the COVID-19 virus targeting the CNS: tissue distribution, host-virus interaction, and proposed neurotropic mechanisms. ACS Chem Neurosci 11(7):995–998. https://doi.org/10.1021/acschemneuro.0c001222
Baskoy K, Ay SA, Altundag A, Kurt O, Salihoglu M, Deniz F, Tekeli H, Yonem A, Hummel T (2016) Is there any effect on smell and taste functions with levothyroxine treatment in subclinical hypothyroidism? PLoS One 11(2):e0149979. https://doi.org/10.1371/journal.pone.0149979
Bhatia-Dey N, Heinbockel T (2020) Endocannabinoid-mediated neuromodulation in the olfactory bulb: functional and therapeutic significance. Int J Mol Sci 21(8):2850. https://doi.org/10.3390/ijms21082850
Bitter T, Gudziol H, Burmeister HP, Mentzel HJ, Guntinas-Lichius O, Gaser C (2010) Anosmia leads to a loss of gray matter in cortical brain areas. Chem Senses 35(5):407–415. https://doi.org/10.1093/chemse/bjq028
Bitter T, Brüderle J, Gudziol H, Burmeister HP, Gaser C, Guntinas-Lichius O (2010) Gray and white matter reduction in hyposmic subjects–a voxel-based morphometry study. Brain Res 6(1347):42–47. https://doi.org/10.1016/j.brainres.2010.06.003
Bougakov D, Podell K, Goldberg E (2020) Multiple neuroinvasive pathways in COVID-19. Mol Neurobiol 58(2):564–575. https://doi.org/10.1007/s12035-020-02152-5
Carod-Artal FJ (2020) Neurological complications of coronavirus and COVID-19. Rev Neurol 70(9):311–322. https://doi.org/10.33588/rn.7009.2020179
Carriere BN, Royal DW, Perrault TJ, Morrison SP, Vaughan JW, Stein BE, Wallace MT (2007) Visual deprivation alters the development of cortical multisensory integration. J Neurophysiol 98(5):2858–2867. https://doi.org/10.1152/jn.00587.2007
Coelho DH, Reiter ER, French E, Costanzo RM (2022) Decreasing incidence of chemosensory changes by COVID-19 variant. Otolaryngol Head Neck Surg. 1945998221097656.https://doi.org/10.1177/01945998221097656
Coolen T, Lolli V, Sadeghi N, Rovai A, Trotta N, Taccone FS, Creteur J, Henrard S, Goffard JC, Dewitte O, Naeije G, Goldman S, De Tiège X (2020) Early postmortem brain MRI findings in COVID-19 non-survivors. Neurology 95(14):e2016–e2027. https://doi.org/10.1212/WNL.0000000000010116
Czesnik D, Schild D, Kuduz J, Manzini I (2007) Cannabinoid action in the olfactory epithelium. Proc Natl Acad Sci USA 104:2967–2972. https://doi.org/10.1073/pnas.0609067104
Derin S, Koseoglu S, Sahin C, Sahan M (2006) Effect of vitamin B12 deficiency on olfactory function. Int Forum Allergy Rhinol 6(10):1051–1055. https://doi.org/10.1002/alr.21790
Dinc ME, Dalgic A, Ulusoy S, Dizdar D, Develioglu O, Topak M (2016) Does iron deficiency anemia affect olfactory function? Acta Otolaryngol 136(7):754–757. https://doi.org/10.3109/00016489.2016.1146410
Doty RL, Bromley SM, Panganiban WD (2006) Olfactory function and dysfunction. In: Bailey BJ, Johnson JT (eds) Head and neck surgery–otolaryngology, 4th edn. Lippincott Williams & Wilkins, Philadelphia, PA, pp 289–305
Hajikhani B, Calcagno T, Nasiri MJ, Jamshidi P, Dadashi M, Goudarzi M, Eshraghi AA, FACS, Mirsaeidi M (2020) Olfactory and gustatory dysfunction in COVID-19 patients: a meta-analysis study. Physiol Rep 8(18):e14578
Han P, Zang Y, Akshita J, Hummel T (2019) Magnetic resonance imaging of human olfactory dysfunction. Brain Topogr 32(6):987–997. https://doi.org/10.1007/s10548-019-00729-5.Erratum.In:BrainTopogr.2019
Hansen BR, Bottner WA, Ravindran A, DeJesus R, Go RS (2017) Desiderosmia (olfactory craving): a novel symptom associated with iron deficiency anemia. Am J Hematol 92(6):E93–E94. https://doi.org/10.1002/ajh.24706
Harapan BN, Yoo HJ (2021) Neurological symptoms, manifestations, and complications associated with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease 19 (COVID-19). J Neurol 268(9):3059–3071. https://doi.org/10.1007/s00415-021-10406-y
Hillard CJ (2018) Circulating endocannabinoids: from whence do they come and where are they going? Neuropsychopharmacology 43(1):155–172. https://doi.org/10.1038/npp.2017.130
Hopkins C, Surda P, Whitehead E, Kumar BN (2020) Early recovery following new onset anosmia during the COVID-19 pandemic: an observational cohort study. J Otolaryngol Head Neck Surg 49:26. https://doi.org/10.1186/s40463-020-00423-8
Hummel T, Landis BN, Hüttenbrink KB (2012) Smell and taste disorders. GMS Curr Top Otorhinolaryngol 10:Doc04. https://doi.org/10.3205/cto000077
Iravani B, Peter MG, Arshamian A, Olsson MJ, Hummel T, Kitzler HH, Lundström JN (2021) Acquired olfactory loss alters functional connectivity and morphology. Scie Rep 11:16422. https://doi.org/10.1038/s41598-021-95968-7
Jothimani D, Kailasam E, Danielraj S, Nallathambi B, Ramachandran H, Sekar P, Manoharan S, Ramani V, Narasimhan G, Kaliamoorthy I, Rela M (2020) COVID-19: poor outcomes in patients with zinc deficiency. Int J Infect Dis 100:343–349. https://doi.org/10.1016/j.ijid.2020.09.014
Kell DB, Pretorius E (2014) Serum ferritin is an important inflammatory disease marker, as it is mainly a leakage product from damaged cells. Metallomics 6(4):748–773. https://doi.org/10.1039/c3mt00347g
Lai CC, Shih TP, Ko WC, Tang HJ, Hsueh PR (2020) Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease-2019 (COVID-19): the epidemic and the challenges. Int J Antimicrob Agents 55(3):105924. https://doi.org/10.1016/j.ijantimicag.2020.105924
Levar N, Francis AN, Smith MJ, Ho WC, Gilman JM (2018) Verbal memory performance and reduced cortical thickness of brain regions along the uncinate fasciculus in young adult cannabis users. Cannabis Cannabinoid Res 3(1):56–65. https://doi.org/10.1089/can.2017.0030
Lin Z, Long F, Yang Y, Chen X, Xu L, Yang M (2020) Serum ferritin as an independent risk factor for severity in COVID-19 patients. J Infect 81(4):647–679. https://doi.org/10.1016/j.jinf.2020.06.053
Lukiw WJ, Pogue A, Hill JM (2022) SARS-CoV-2 infectivity and neurological targets in the brain. Cell Mol Neurobiol 42(1):217–224. https://doi.org/10.1007/s10571-020-00947-7
Maccarrone M, Bab I, Bíró T, Cabral GA, Dey SK, Di Marzo V, Konje JC, Kunos G, Mechoulam R, Pacher P, Sharkey KA, Zimmer A (2015) Endocannabinoid signaling at the periphery: 50 years after THC. Trends Pharmacol Sci 36(5):277–296. https://doi.org/10.1016/j.tips.2015.02.008
Mackay-Sim A, Beard MD (1987) Hypothyroidism disrupts neural development in the olfactory epithelium of adult mice. Brain Res 36(2):190–198
Magusali N, Graham AC, Piers TM, Panichnantakul P, Yaman U, Shoai M, Reynolds RH, Botia JA, Brookes KJ, Guetta-Baranes T, Bellou E, Bayram S, Sokolova D, Ryten M, Sala Frigerio C, Escott-Price V, Morgan K, Pocock JM, Hardy J, Salih DA (2021) A genetic link between risk for Alzheimer’s disease and severe COVID-19 outcomes via the OAS1 gene. Brain 144(12):3727–3741. https://doi.org/10.1093/brain/awab337
Marinosci A, Landis BN, Calmy A (2020) Possible link between anosmia and COVID-19: sniffing out the truth. Eur Arch Otorhinolaryngol 277(7):2149–2150. https://doi.org/10.1007/s00405-020-05966-0
Mueller A, Rodewald A, Reden J, Gerber J, von Kummer R, Hummel T (2005) Reduced olfactory bulb volume in post-traumatic and post-infectious olfactory dysfunction. Neuro Report 16:475–478. https://doi.org/10.1097/00001756-200504040-00011
Ou X, Liu Y, Lei X, Li P, Mi D, Ren L, Guo L, Guo R, Chen T, Hu J, Xiang Z, Mu Z, Chen X, Chen J, Hu K, Jin Q, Wang J, Qian Z (2020) Characterization of spike glycoprotein of SARS-CoV-2 on virus entry and its immune cross-reactivity with SARS-CoV. Nat Commun 11:1620. https://doi.org/10.1038/s41467-020-15562-9
Petekkaya E, Kuş B, Doğan S, Bayaroğulları H, Mutlu T, Melek IM, Arpacı A (2022) Possible role of endocannabinoids in olfactory and taste dysfunctions in Alzheimer’s and Parkinson’s patients and volumetric changes in the brain. J Clin Neurosci 100:52–58. https://doi.org/10.1016/j.jocn.2022.03.047
Pourbagheri-Sigaroodi A, Bashash D, Fateh F, Abolghasemi H (2020) Laboratory findings in COVID-19 diagnosis and prognosis. Clin Chim Acta 510:475. https://doi.org/10.1016/j.cca.2020.08.019
Reiken S, Sittenfeld L, Dridi H, Liu Y, Liu X, Marks AR (2022) Alzheimer’s-like signaling in brains of COVID-19 patients. Alzheimers Dement. https://doi.org/10.1002/alz.12558.10.1002/alz.12558
Sinclair J (2016) An introduction to cannabis and the endocannabinoid system. Aust J Herb Med 28(4):107–125
Strauss SB, Lantos JE, Heier LA, Shatzkes DR, Phillips CD (2020) Olfactory bulb signal abnormality in patients with COVID-19 who present with neurologic symptoms. Am J Neuroradiol 41(10):1882–1887. https://doi.org/10.3174/ajnr.A6751
Świdziński T, Linkowska-Świdzińska K, Czerniejewska-Wolska H, Wiskirska-Woźnica B, Owecki M, Głowacka MD, Frankowska A, Łącka K, Glapiński M, Maciejewska-Szaniec Z, Świdziński P (2016) Hypothyroidism affects olfactory evoked potentials. Biomed Res Int 9583495.https://doi.org/10.1155/2016/9583495
Tomita H (1990) Zinc in taste and smell disorders. In Trace elements in clinical medicine Springer, Tokyo, 15–37
Wang ZJ, Sun L, Heinbockel T (2012) Cannabinoid receptor-mediated regulation of neuronal activity and signaling in glomeruli of the main olfactory bulb. J Neurosci 32:8475–8479. https://doi.org/10.1523/JNEUROSCI.5333-11.2012
Wang T, Du Z, Zhu F, Cao Z, An Y, Gao Y, Jiang B (2020) Comorbidities and multi-organ injuries in the treatment of COVID-19. Lancet 395(10228):e52. https://doi.org/10.1016/S0140-6736(20)30558-4
Xu H, Zhong L, Deng J, Peng J, Dan H, Zeng X, Li T, Chen Q (2020) High expression of ACE2 receptor of 2019-nCoV on the epithelial cells of oral mucosa. Int J Oral Sci 12(1):8. https://doi.org/10.1038/s41368-020-0074-x
Funding
This work was supported by Kastamonu University Scientific Research Projects Coordination Unit (project number KÜ-BAP01/2021–11).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation and data collection and analysis were performed by Zafer Ergül, Zülal Kaptan, Ayhan Kars, Gülşah Biçer, Çetin Kılınç, Emine Petekkaya, and Nilay Çöplü. The first draft of the manuscript was written by Zülal Kaptan and Emine Petekkaya, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethical Approval
This study was performed in line with the principles of the Declaration of Helsinki. The questionnaire and methodology for this research were approved at the meeting of Kastamonu University Clinical Research Ethics Committee on 26/11/2020 (decision no: 2020-KAEK-143–13).
Informed Consent
Informed consent was obtained from all individual participants included in the study. Patients signed informed consent regarding publishing their data and photographs.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ergül, Z., Kaptan, Z., Kars, A. et al. Possible Role of Endocannabinoids in Olfactory and Taste Dysfunctions in COVID-19 Patients and Volumetric Changes in the Brain. Chem. Percept. 15, 135–144 (2022). https://doi.org/10.1007/s12078-022-09301-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12078-022-09301-1