Abstract
Glycophorins are transmembrane proteins of red blood cells (RBCs), heavily glycosylated on their external-facing surface. In humans, there are four glycophorin proteins, glycophorins A, B, C and D. Glycophorins A and B are encoded by two similar genes GYPA and GYPB, and glycophorin C and glycophorin D are encoded by a single gene, GYPC. The exact function of glycophorins remains unclear. However, given their abundance on the surface of RBCs, it is likely that they serve as a substrate for glycosylation, giving the RBC a negatively charged, complex glycan “coat”. GYPB and GYPE (a closely related pseudogene) were generated from GYPA by two duplication events involving a 120-kb genomic segment between 10 and 15 million years ago. Non-allelic homologous recombination between these 120-kb repeats generates a variety of duplication alleles and deletion alleles, which have been systematically catalogued from genomic sequence data. One allele, called DUP4, encodes the Dantu NE blood type and is strongly protective against malaria as it alters the surface tension of the RBC membrane. Glycophorins interact with other infectious pathogens, including viruses, as well as the malarial parasite Plasmodium falciparum, but the role of glycophorin variation in mediating the effects of these pathogens remains underexplored.
Similar content being viewed by others
Glycophorin genes and function
Glycophorins are transmembrane proteins of red blood cells (RBCs), heavily glycosylated on their external-facing surface. In humans, there are four glycophorin proteins, glycophorins A, B, C and D. Glycophorins A and B are encoded by two similar genes GYPA and GYPB, and glycophorin C and glycophorin D are encoded by a single gene, GYPC, which is not related to GYPA/GYPB. Glycophorin C and glycophorin D differ due to different translational start sites on the GYPC transcript (Le Van et al. 1987). A gene annotated as GYPE, which is very similar to GYPA and GYPB, is transcribed, but no protein product for glycophorin E has been detected; therefore, GYPE is likely to be a pseudogene (Fig. 1, Vignal et al. 1990). Glycophorins have been characterised as carrying the antigens for several human blood groups. Glycophorins A and B carry the MN and Ss blood groups, and glycophorin C carries the Gerbich blood group system (Daniels 2008; Lopez et al. 2021). Rare individuals without glycophorin A (En), glycophorin B (S- s- U-) or both (Mk) have been identified by the absence of particular blood groups carried by these proteins. Individuals who lack glycophorin B or glycophorin A are healthy (Tokunaga et al. 1979), so the exact function of these glycophorins remains unclear. Glycophorin C has been shown to have a role in maintaining the biconcave discoid shape of the RBC (Reid et al. 1987). Given their abundance of glycophorins on the surface of RBCs, it is likely that they also serve as a substrate for glycosylation, giving the RBC a negatively charged, complex glycan “coat” allowing circulation without adherence to other cells or walls of blood vessels.
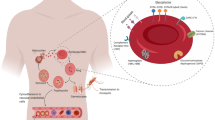
Summary of the role of glycophorins and infectious disease. Overview of the central concepts discussed in this review. Structural variation affects two distinct loci carrying the GYPC gene and GYPA/GYPB/GYPE. Different variants encode distinct glycophorin variants on the red blood cell surface. These glycophorins interact with a variety of different pathogens, including viruses, bacteria and malaria. Created with Biorender.com
Evolution of glycophorin genes in primates
Primates, and other mammals, have a single GYPA gene, with the exception of bonobos, chimpanzees, gorillas and humans, which all have three related genes (GYPA, GYPB and GYPE) (Rearden et al. 1993), sharing about 97% identity. These three genes were generated by two duplication events after divergence of orangutans but before divergence of gorillas from the human lineage (about 10–15 MYA) (Fig. 2a; Kudo and Fukuda 1990; Rearden et al. 1993). There is no evidence for duplication of GYPC, as all primates have a single GYPC gene (Wilder et al. 2009). However, the translation initiation codon for glycophorin C appears to be specific to humans, with the translation initiation codon for glycophorin D conserved across apes. Glycophorin C is therefore a human-specific protein, with glycophorin D being present in all apes (Wilder et al. 2009).
Evolution of glycophorins in great apes. a The tree shows the phylogeny of great apes, with branches annotated with the changes in glycophorin genes along the branches. b Fibre-FISH representative image of the human glycophorin region showing the reference haplotype. 120-kb repeats carrying GYPE, GYPB and GYPA are represented by coloured bars green, orange and purple, respectively. Each one of the genes was identified by a specific FISH pattern using region-specific fosmid clones (details in Louzada et al. 2020). c Structural variation in the glycophorin region in chimpanzee revealed by fibre-FISH, highlighting the presence of three copies of GYPE (fibre-FISH details in Louzada et al. 2020)
Structural variation of glycophorin genes
The glycophorin genes A, B and E are on approximately 120-kb tandemly arranged repeats on chromosome 4 (Fig. 2b), and, because of this, are prone to rearrangements driven by recurrent non-allelic homologous recombination (NAHR) events. These events can be either deletions or duplications, and involve either GYPA-GYPE, GYPA-GYPB or GYPB-GYPE as partners. More complex events can be generated, most likely the result of a series of individual NAHR events. If the complex events involve the regions where the glycophorin genes are encoded, then fusion genes can be formed from different exons of GYPA/GYPE and GYPB. Many of these variants were initially detected as novel, rare, blood groups (Daniels 2008). Analysis of the molecular genetic basis of particular rare blood groups (e.g. some alleles with the S- s- blood group, Willemetz et al. 2015) has shown that gene conversion, where a region from one gene is “copied and pasted” into another, is a further source of genetic variation. Because GYPC is in a single copy region on chromosome 2, the gene is not prone to extensive complex structural variation; however, the Gerbich negative blood types are caused by small deletions of exon 2 (Ge2), exon 3 (Ge3) or both exons 2 and 3 (Ge4) and the Gerbich Lsa antigen is caused by a duplication or triplication of GYPC exon 3 (Jaskiewicz et al. 2018).
Genome sequence data has allowed a systematic cataloguing of structural variants across the region (Leffler et al. 2017). Many have been validated by fibre-FISH, breakpoint PCR, and some have been shown to underlie blood group variation (Louzada et al. 2020). The sizes of the observed duplications and deletions usually correspond to loss or gain of one, or sometimes two, repeat units of ~ 120 kb each. The most complex structural variant yet identified is DUP4, which is the molecular basis of the Dantu NE blood group. This is partial duplication/triplication and generates loss of GYPB but a duplication of GYPE and three copies of a novel GYPB-GYPA fusion gene which is expressed on the RBC surface (Leffler et al. 2017; Algady et al. 2018). In contrast to structural variants that cause changes in copy number, a systematic exploration of gene conversion variants and inversion variants in the region is lacking. Given the challenges in mapping short sequence reads to duplicated regions such as the glycophorin A-B-E region, accurate long sequence reads will be needed to robustly distinguish gene conversion events from sequence read mis-mapping.
Compared to humans, little is known about structural variation in primate glycophorins. Genome assemblies using long-read sequence data give an indication of at least one structural arrangement of the region, for example the latest bonobo assembly (Mao et al. 2021, panPan3) shows the same glycophorin arrangement as humans. However, the genome region containing glycophorin A-B-E is currently incompletely assembled in the most recent gorilla assembly (ggor6) and chimpanzee assembly (Clint_PTRv2), presumably because of its highly duplicated structure. Although the A-B-E genomic structure has been confirmed in gorillas (Xie et al. 1997), we have observed a gorilla with a total of four glycophorin genes using fibre-FISH, though we were unable to determine whether the extra glycophorin gene was GYPA, GYPB or GYPE (Louzada, Hollox and Yang unpublished). It is known that GYPE is polymorphic in copy number in gorillas, being completely absent in 9/16 individuals (~ 56%) (Rearden et al. 1993), so the extra gene we observe is likely to be GYPE. In an early chimpanzee reference genome (panTro2), three GYPE genes were annotated (Ko et al. 2011), and this arrangement confirmed using fibre-FISH (Fig. 2c). It is likely that other genes, beyond GYPE, will be copy number variable in chimpanzees and gorillas, but a comprehensive study is needed.
Genotyping the variation in glycophorin genes
Although genome sequencing is becoming cheaper and more cost effective, there is still an important role for methods designed to genotype structural variants by PCR, particularly for limited samples or in situations with limited resources. For DUP4, methods involving PCR amplification of GYPB-GYPA and GYPA followed by restriction enzyme digestion and gel electrophoresis to distinguish the genes (Leffler et al. 2017) or breakpoint-specific PCR (Algady et al. 2018) have been developed. Designing a PCR spanning the SV breakpoint is challenging because PCR primers are designed to be specific not only to the allele but the paralogue as well. However, for other variants, in particular GYPB deletion alleles, breakpoint-specific PCRs and PCR-based paralogue ratio tests have been developed (Lane et al. 2020; Algady et al. 2021; Amuzu et al. 2021).
For genotyping single nucleotide variation, mis-mapping of short sequencing reads between paralogues can limit accuracy, particularly in regions where gene conversion alleles have occurred. Similarly, paralogues need to be distinguished in PCR approaches by carefully validating the paralogue-specificity of PCR primers, to ensure the correct locus is genotyped. As for structural variation, long read sequencing will make accurate genotyping of these duplicated regions more reliable, and allow for improvements in haplotype phasing of variants.
Glycophorins in malaria
Both glycophorin A and glycophorin B act as receptors EBA-175 and EBL-1 on the surface of Plasmodium falciparum, the parasite responsible for malaria in Africa. Glycophorin C also interacts with P. falciparum through its EBA-140 receptor (Wassmer and Carlton 2016). The DUP4 structural variant, encoding the Dantu blood group, has been shown to be protective against severe malaria, with homozygotes showing 74% protection against severe malaria (Field et al. 1994; Leffler et al. 2017). Furthermore, in a village-based non-hospital setting with endemic P. falciparum malaria, DUP4 has been shown to be associated with higher haemoglobin levels, likely reflecting DUP4 protection against malarial anaemia (Algady et al. 2018). DUP4 protects against malaria not by altering ligand-receptor interactions with P. falciparum, but by increasing the RBC surface tension preventing P. falciparum invasion (Kariuki et al. 2020).
Despite functional evidence showing that RBCs completely lacking glycophorins A and B are partially resistant to P. falciparum invasion (Hadley et al. 1987), there is no genetic evidence suggesting that other alleles of the glycophorin A-B-E region affect susceptibility to malaria. A functional study suggested that an allele at GYPC encoding Gerbich negative blood types, and at high frequency in Melanesians, was protective against P. falciparum invasion (Maier et al. 2003); however, there is no support for this from recent large-scale association studies in other populations.
Malaria is known to have been a major agent of natural selection in humans who live where malaria is endemic. Because of the role of glycophorins in malaria, there are several studies that assess genetic variation for signs of natural selection, and discover evidence for natural selection at the glycophorin A-B-E region (Baum et al. 2002; Ko et al. 2011; Bigham et al. 2018; Johnson and Voight 2018). Although this is consistent with our expectations, methods using sequence diversity and divergence may be biased because of the highly duplicated nature of the glycophorin A-B-E region, and the extensive recombination, copy number variation and gene conversion that occurs. More recent selection can be detected using an extended haplotype test, which compares LD with allele frequency to test for strong recent positive selection of a variant, and is likely to underestimate selection in the presence of gene conversion. The DUP4 variant is young as it is restricted to East Africa (Table 1, Leffler et al. 2017). Using the extended haplotype test, it has been shown that DUP4 has undergone recent positive selection to rapidly increase in frequency, presumably due to its protective effect against malaria (Leffler et al. 2017).
Unlike glycophorins A, B and E, the GYPC gene is not the result of a recent duplication, and lacks close paralogues. Comparative evolutionary studies are therefore more straightforward as the correct orthologue can be confidently identified, and analysis of genetic diversity is not affected by potential mis-mapping of sequence reads. Comparative analysis has shown that glycophorins C and D, encoded by GYPC, have undergone recent natural selection of the extracellular domain, strongly suggesting pathogen-mediated evolution (Wilder et al. 2009).
Glycophorins in other infectious diseases
There is some evidence that glycophorins A and B act as receptors for other pathogens. Babesia divergens, which, like Plasmodium, is a member of the Apicomplexa phylum, is an eukaryotic intracellular parasite which can cause malarial-like symptoms in immunocompromised humans, uses glycophorins A and B to enter the RBC (Lobo 2005).
Some strains of Escherichia coli bind to glycophorin A on the surface of RBCs (Cooling 2015), and glycophorin A acts as the receptor to reoviruses, double stranded RNA viruses which include the rotavirus family. The single stranded RNA viruses encephalomyocarditis virus and hepatitis A also seem to use glycophorin A as a receptor for infection. Influenza viruses have been shown to interact with glycophorin A, and because influenza viruses cannot replicate in the anucleated RBC, it has been suggested that glycophorins act as decoy receptors diverting infection away from other tissues (Gagneux and Varki 1999).
Conclusion
Glycophorins are major glycoproteins of the RBC surface, and are receptors for the malarial parasite P. falciparum. The region containing three paralogous 120-kb repeats, carrying the GYPA, GYPB and GYPE genes, has been generated by repeated rounds of duplication between 10 and 15 MYA, and shows extensive complex structural variation. One structural variant, DUP4, encodes the Dantu blood group antigen and is strongly protective against severe malaria. The role of genetic variation in the response to other pathogens that use glycophorins as receptors remains unclear.
References
Algady W, Louzada S, Carpenter D et al (2018) The malaria-protective human glycophorin structural variant DUP4 shows somatic mosaicism and association with hemoglobin levels. Am J Hum Genet 103:769–776
Algady W, Weyell E, Mateja D et al (2021) Genotyping complex structural variation at the malaria-associated human glycophorin locus using a PCR-based strategy. Ann Hum Genet 85:7–17
Amuzu DS, Rockett KA, Leffler EM et al (2021) High-throughput genotyping assays for identification of glycophorin B deletion variants in population studies. Exp Biol Med 246:916–928
Baum J, Ward RH, Conway DJ (2002) Natural selection on the erythrocyte surface. Mol Biol Evol 19:223–229
Bigham AW, Magnaye K, Dunn DM et al (2018) Complex signatures of natural selection at GYPA. Hum Genet 137:151–160
Cooling L (2015) Blood groups in infection and host susceptibility. Clin Microbiol Rev 28:801–870
Daniels G (2008) Human blood groups. John Wiley & Sons
Field SP, Hempelmann E, Mendelow BV, Fleming AF (1994) Glycophorin variants and Plasmodium falciparum: protective effect of the Dantu phenotype in vitro. Hum Genet 93:148–150
Gagneux P, Varki A (1999) Evolutionary considerations in relating oligosaccharide diversity to biological function. Glycobiology 9:747–755
Hadley TJ, Klotz FW, Pasvol G et al (1987) Falciparum malaria parasites invade erythrocytes that lack glycophorin A and B (MkMk). Strain differences indicate receptor heterogeneity and two pathways for invasion. J Clin Invest 80:1190–1193
Jaskiewicz E, Peyrard T, Kaczmarek R et al (2018) The Gerbich blood group system: old knowledge, new importance. Transfus Med Rev 32:111–116
Johnson KE, Voight BF (2018) Patterns of shared signatures of recent positive selection across human populations. Nat Ecol Evol 2:713–720
Kariuki SN, Marin-Menendez A, Introini V et al (2020) Red blood cell tension protects against severe malaria in the Dantu blood group. Nature 585:579–583
Ko W-Y, Kaercher KA, Giombini E et al (2011) Effects of natural selection and gene conversion on the evolution of human glycophorins coding for MNS blood polymorphisms in malaria-endemic African populations. Am J Hum Genet 88:741–754
Kudo S, Fukuda M (1990) Identification of a novel human glycophorin, glycophorin E, by isolation of genomic clones and complementary DNA clones utilizing polymerase chain reaction. J Biol Chem 265:1102–1110
Lane WJ, Gleadall NS, Aeschlimann J et al (2020) Multiple GYPB gene deletions associated with the U- phenotype in those of African ancestry. Transfusion (paris) 60:1294–1307
Le Van KC, Colin Y, Blanchard D et al (1987) Gerbich blood group deficiency of the Ge:-1,-2,-3 and Ge:-1,-2,3 types. Immunochemical study and genomic analysis with cDNA probes. Eur J Biochem 165:571–579
Leffler EM, Band G, Busby GBJ, et al (2017) Resistance to malaria through structural variation of red blood cell invasion receptors. Science 356(6343)
Lobo C-A (2005) Babesia divergens and plasmodium falciparum use common receptors, glycophorins A and B, to invade the human red blood cell. Infect Immun 73(1):649–651
Lopez GH, Hyland CA, Flower RL (2021) Glycophorins and the MNS blood group system: a narrative review. Ann Blood 6:39
Louzada S, Algady W, Weyell E et al (2020) Structural variation of the malaria-associated human glycophorin A-B-E region. BMC Genomics 21:446
Maier AG, Duraisingh MT, Reeder JC et al (2003) Plasmodium falciparum erythrocyte invasion through glycophorin C and selection for Gerbich negativity in human populations. Nat Med 9:87–92
Mao Y, Catacchio CR, Hillier LW et al (2021) A high-quality bonobo genome refines the analysis of hominid evolution. Nature 594:77–81
Moores P, Smart E, Marais I (1992) The Dantu phenotype in Southern Africa. Transfus Med. 2 Supp 1:68 (abstract)
Rearden A, Magnet A, Kudo S, Fukuda M (1993) Glycophorin B and glycophorin E genes arose from the glycophorin A ancestral gene via two duplications during primate evolution. J Biol Chem 268:2260–2267
Reid ME, Chasis JA, Mohandas N (1987) Identification of a functional role for human erythrocyte sialoglycoproteins β and γ. Blood 69:1068–1072
Tokunaga E, Sasakawa S, Tamaka K et al (1979) Two apparently healthy Japanese individuals of type MkMk have erythrocytes which lack both the blood group MN and Ss-active sialoglycoproteins. Int J Immunogenet 6:383–390
Unger P, Procter JL, Moulds JJ et al (1987) The Dantu erythrocyte phenotype of the NE variety. II. Serology, immunochemistry, genetics, and frequency. Blut 55:33–43
Vignal A, Rahuel C, London J et al (1990) A novel gene member of the human glycophorin A and B gene family. Molecular cloning and expression. Eur J Biochem 191:619–625
Wassmer SC, Carlton JM (2016) Glycophorins, blood groups, and protection from severe malaria. Trends Parasitol 32:5–7
Wilder JA, Hewett EK, Gansner ME (2009) Molecular evolution of GYPC: evidence for recent structural innovation and positive selection in humans. Mol Biol Evol 26:2679–2687
Willemetz A, Nataf J, Thonier V et al (2015) Gene conversion events between GYPB and GYPE abolish expression of the S and s blood group antigens. Vox Sang 108:410–416
Xie SS, Huang CH, Reid ME et al (1997) The glycophorin A gene family in gorillas: structure, expression, and comparison with the human and chimpanzee homologues. Biochem Genet 35:59–76
Acknowledgements
We acknowledge the many colleagues who have contributed to our knowledge of glycophorins and whose work could not be cited because of space limitations.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hollox, E.J., Louzada, S. Genetic variation of glycophorins and infectious disease. Immunogenetics 75, 201–206 (2023). https://doi.org/10.1007/s00251-022-01280-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00251-022-01280-7