Abstract
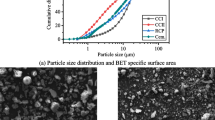
Red mud is another worldwide problem after the bulk solid waste of steel slag.The reaction time between phases of aluminate cement clinker in the molten state is approximately 20 min, and the phase composition obtained is CA. After quenching, a large area of the glass phase appeared in the SEM images of the aluminate cement clinker. The compressive strength and flexural strength at 28 days reached 77.7 and 7.6 MPa, respectively, and the hydration strengths at 1, 3, and 28 days were higher than the strength standard of CA50II aluminate cement.Reconstruction of iron extraction tailings from red mud melting reduction is an effective technology to solve the problems of low cementitious activity, complex composition and large chemical fluctuation of red mud, and it has become an effective technology to improve the comprehensive utilization rate of red mud and promote energy savings and emission reduction.










Similar content being viewed by others
REFERENCES
Liu, W., Yang, J., and Xiao, B., Review on treatment and utilization of bauxite residues in China, Int. J. Miner. Process., 2009, vol. 93, pp. 220–231.
Li, L.Y., Properties of red mud of tailings produced under varying process conditions, J. Environ. Eng., 1998, vol. 124, no. 3, pp. 254–264.
Liu, Y. and Naidu, R., Hidden values in bauxite residue (red mud): recovery of metals, Waste Manage., 2014, vol. 34, pp. 2662–2673.
Zhang, T.A., Lv, G.Z., Liu, Y., et al., CN Patent CN201110275030.X, 2011.
Zhang, T.A., Lv, G.Z., Liu, Y., et al., CN Patent CN201610333963.2, 2016.
Kumar, S., Kumar, R., and Bandopadhyay, A., Innovative methodologies for the utilization of wastes from metallurgical and allied industries, Resour., Conserv. Recycl., 2006, vol. 48, no. 4, pp. 301–314.
Zhang, Ta., Wang, Y.X., Lu, G.Z., Liu, Y., Zhang, W.G., and Zhao, Q.Y., Comprehensive utilization of red mud: current research status and a possible way forward for non-hazardous treatment, in Light Metals 2018, The Minerals, Metals and Materials Society Series, Ratvik, A.P., Ed., New York: Springer, 2018, pp. 135–141.
Li, L.Y., A study of iron mineral transformation to reduce red mud tailings, Waste Manage., 2001, vol. 21, no. 6, pp. 525−534.
Wang, K., Liu, Y., Zhang, T.A., Li, X.F., and Chen, X., Investigation of the smelting reduction mechanism and of iron extraction from high-iron red mud, Mater. Res. Express, 2020, vol. 7, p. 126514.
Wang, K., Liu, Y., Li, X., Dou, Z., Lu, G., and Zhang, T., Production of pig iron from high-iron red mud by smelting reduction, in Light Metals, Eskin, D., Ed., Springer, 2022, pp. 41–47.
Loginova, I.V., Shoppert, A.A., Kyrchikov, A.V., et al., Using iron-rich red mud from alumina production at steel plants, Steel Transl., 2016, vol. 46, pp. 74–77.
Arslan, S., Ucbeyiay, H., Çelikel, B., Baygül, M., Avcu, S., and Demir, G.K., Eti aluminum red mud characteristics and evaluation of dewatering performance, Proc. Bauxite Residue Valorization and Best Practices Conference-2015, Leuven: KU Leuven, October 5–7, 2015.
Ercag, E. and Apak, R., Furnace smelting and extractive metallurgy of red mud: recovery of TiO2, Al2O3 and pig iron, J. Chem. Technol. Biotechnol., 1997, vol. 70, pp. 241–246.
Wang, K., Liu, Y., Lyu, G., Li, X., Chen, X., and Zhang, T., Recovery of iron from high-iron Bayer red mud by smelting reduction, in Light Metals 2020, The Minerals, Metals and Materials Series, Tomsett, A., Ed., Cham: Springer, 2020. https://doi.org/10.1007/978-3-030-36408-3_13
Ren, C.Z., Wang, W.L., Mao, Y.P., Yuan, X.L., Song, Z.L., Sun, J., and Zhao, X.Q., Comparative life cycle assessment of sulfoaluminate clinker production derived from industrial solid wastes and conventional raw materials, J. Cleaner Prod., 2017, vol. 167, no. 20, pp. 1314–1324.
Guo, Y.H., Gao, J.J., Xu, H.J., Zhao, K., and Shi, X.F., Nuggets production by direct reduction of high iron red mud, J. Iron Steel Res. Int., 2013, vol. 20, no. 5, pp. 24–27.
Valeev, D., Zinoveev, D., Kondratiev, A., Lubyanoi, D., and Pankratov, D., Reductive smelting of neutralized red mud for iron recovery and produced pig iron for heat-resistant castings, Metals, 2020, vol. 10, p. 32.
Gu, H., Hargreaves, J.S.J., Jiang, J.Q., and Rico, J.L., Potential routes to obtain value-added iron-containing compounds from red mud, J. Sustainable Metall., 2017, vol. 3, no. 3, pp. 561−569.
Liu, X., Zhang, N., Sun, H., Zhang, J., and Li, L., Structural investigation relating to the cementitious activity of bauxite residue—red mud, Cem. Concr. Res., 2011, vol. 41, pp. 847–853.
Samal, S., Ray, A.K., and Bandopadhyay, A., Characterization and microstructure observation of sintered red mud-fly ash mixture at various elevated temperature, J. Cleaner Prod., 2015, vol. 101, pp. 368–376.
Wang, X., Luo, Z.T., Zhang, L., Rong, H., and Yang, J.J., Utilization of red mud as raw material in the production of field road cement, J. Wuhan Univ. Technol., 2016, vol. 31, pp. 877–882.
Thakur, R.S. and Sant, B.R., Utilization of red mud, part I analysis and utilization of raw material for absorbents, building materials, catalysts, fillers, paints and pigments, J. Sci. Ind. Res., 1983, vol. 42, pp. 87–108.
Mishra, C.R., Yadav, D., Sharma, P.S., and Alli, M.M., Production of Ordinary Portland Cement (OPC) from Nalco Red Mud, in Light Metals, Lindsay, S.J., Ed., Cham: Springer, 2011. https://doi.org/10.1007/978-3-319-48160-9_17
Singh, M., Upadhayay, S.N., and Prasad, P.M., Preparation of special cements from red mud, Waste Manage, 1996, vol. 16, pp. 665–670.
Kang, S.P., Properties of alkali-activated slag-red mud soil pavement using recycled aggregate, J. Korean Recycled Constr. Resour. Inst., 2016, vol. 4, no. 3, pp. 276–283.
Li, R.B., Zhang, T.A., Liu, Y., Lv, G.Z., and Xie L.Q., Calcification—carbonation method for red mud processing, J. Hazard. Mater., 2016, vol. 316, pp. 94–101.
Zhang, T., Wang, K., Liu, Y., Lyu, G., Li, X., and Chen, X., A Review of comprehensive utilization of high-iron red mud of China, in Light Metals, 2020, The Minerals, Metals and Materials Series, Tomsett, A., Ed., Cham: Springer, 2020. https://doi.org/10.1007/978-3-030-36408-3_10
Li, X.B., Xiao, W., Liu, W., Liu, G.H., Peng, Z.H., Zhou, Q.S., and Qi, T.G., Recovery of alumina and ferric oxide from Bayer red mud rich in iron by reduction sintering, Trans. Nonferrous Met. Soc. China, 2009, vol. 19, pp. 1342–1347.
Kang, S.P. and Kwon, S.J., Effects of red mud and alkali-activated slag cement on efflorescence in cement mortar, Constr. Build. Mater., 2017, vol. 133, pp. 459–467.
Al-Akhras, N.M. and Smadi, M.N., Properties of tire rubber ash mortar, Cem. Concr. Compos., 2004, vol. 26, pp. 821–826.
Gonga, C. and Yang, N., Effect of phosphate on the hydration of alkali-activated red mud slag cementitious material., Cem. Concr. Res., 2000, vol. 30, pp. 1013–1016.
Pan, Z., Cheng, L., Lu, Y., and Yang, N., Hydration products of alkali-activated slag-red mud cementitious material., Cem. Concr. Res., 2002, vol. 32, pp. 357–362.
Hyeok-Jung, K., Kang, S.P., and Choe, G.C., Effect of red mud content on strength and efflorescence in pavement using alkali-activated slag cement, Int. J. Concr. Struct. Mater., 2018, vol. 12, p. 18. https://doi.org/10.1186/s40069-018-0258-3
ACKNOWLEDGMENTS
Thanks to the Key Laboratory of Ecological Utilization of Multimetal Intergrown Ores of Ministry of Education of Northeastern University for training and educating,Thanks to my teacher Zhang Tingan for his careful guidance, Thanks for helping me have a deeper understanding of the experimental ideas and the subject, thanks to Ye Jiayuan, Dean of the State Key Laboratory of Green Building Materials of China National Building Materials Group, and Zhang Jinshan for their technical support and help, thanks to Lv Guozhi for his words and deeds, and thanks to my team brothers for their companionship and care.
Funding
This research is supported by the National Natural Science Foundation of China (nos. U1903129, U1710257, 51874078, U1202274).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest.
About this article
Cite this article
Xuewei, Y., Xin, C., Ting’an, Z. et al. Study on the Cementitious Properties of Aluminate Cement Clinker Prepared from Melt Reduction Slag of Quenched and Tempered High-Iron Red Mud. Russ. J. Non-ferrous Metals 63, 500–509 (2022). https://doi.org/10.3103/S106782122205011X
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.3103/S106782122205011X