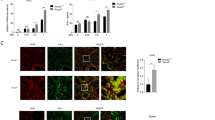
Abstract
It has been reported that IL-33 receptor ST2 deficiency mitigates Cryptococcus neoformans (C. neoformans) pulmonary infection in BALB/c mice. IL-33 may modulate immune responses in ST2-dependent and ST2-independent manners. The host genetic background (i.e., BALB/c, C57BL/6 J) influences immune responses against C. neoformans. In the present study, we aimed to explore the roles of IL-33 and ST2 in pulmonary C. neoformans-infected mice on a C57BL/6 J genetic background. C. neoformans infection increased IL-33 expression in lung tissues. IL-33 deficiency but not ST2 deficiency significantly extended the survival time of C. neoformans-infected mice. In contrast, either IL-33 or ST2 deficiency reduced fungal burdens in lung, spleen and brain tissues from the mice following C. neoformans intratracheal inoculation. Similarly, inflammatory responses in the lung tissues were more pronounced in both the IL-33−/− and ST2−/− infected mice. However, mucus production was decreased in IL-33−/− infected mice alone, and the level of IL-5 in bronchoalveolar lavage fluid (BALF) was substantially decreased in the IL-33−/− infected mice but not ST2−/− infected mice. Moreover, IL-33 deficiency but not ST2 deficiency increased iNOS-positive macrophages. At the early stage of infection, the reduced pulmonary fungal burden in the IL-33−/− and ST2−/− mice was accompanied by increased neutrophil infiltration. Collectively, IL-33 regulated pulmonary C. neoformans infection in an ST2-dependent and ST2-independent manner in C57BL/6 J mice.






Similar content being viewed by others
Data availability
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.
References
Zhang Y, Wang F, Tompkins KC, McNamara A, Jain AV, Moore BB et al (2009) Robust Th1 and Th17 immunity supports pulmonary clearance but cannot prevent systemic dissemination of highly virulent Cryptococcus neoformans H99. Am J Pathol 175(6):2489–2500
Jain AV, Zhang Y, Fields WB, McNamara DA, Choe MY, Chen GH et al (2009) Th2 but not Th1 immune bias results in altered lung functions in a murine model of pulmonary Cryptococcus neoformans infection. Infect Immun 77(12):5389–5399
Chen GH, McNamara DA, Hernandez Y, Huffnagle GB, Toews GB, Olszewski MA (2008) Inheritance of immune polarization patterns is linked to resistance versus susceptibility to Cryptococcus neoformans in a mouse model. Infect Immun 76(6):2379–2391
Heyen L, Muller U, Siegemund S, Schulze B, Protschka M, Alber G et al (2016) Lung epithelium is the major source of IL-33 and is regulated by IL-33-dependent and IL-33-independent mechanisms in pulmonary cryptococcosis. Pathog Dis 74(7):ftw086
Li D, Guabiraba R, Besnard AG, Komai-Koma M, Jabir MS, Zhang L et al (2014) IL-33 promotes ST2-dependent lung fibrosis by the induction of alternatively activated macrophages and innate lymphoid cells in mice. J Allergy Clin Immunol 134(6):1422–32.e11
Flaczyk A, Duerr CU, Shourian M, Lafferty EI, Fritz JH, Qureshi ST (2013) IL-33 signaling regulates innate and adaptive immunity to Cryptococcus neoformans. J Immunol 191(5):2503–2513
Piehler D, Grahnert A, Eschke M, Richter T, Kohler G, Stenzel W et al (2013) T1/ST2 promotes T helper 2 cell activation and polyfunctionality in bronchopulmonary mycosis. Mucosal Immunol 6(2):405–414
Piehler D, Eschke M, Schulze B, Protschka M, Muller U, Grahnert A et al (2016) The IL-33 receptor (ST2) regulates early IL-13 production in fungus-induced allergic airway inflammation. Mucosal Immunol 9(4):937–949
Alvarez F, Istomine R, Shourian M, Pavey N, Al-Aubodah TA, Qureshi S et al (2019) The alarmins IL-1 and IL-33 differentially regulate the functional specialisation of Foxp3(+) regulatory T cells during mucosal inflammation. Mucosal Immunol 12(3):746–760
Lefrançais E, Duval A, Mirey E, Roga S, Espinosa E, Cayrol C et al (2014) Central domain of IL-33 is cleaved by mast cell proteases for potent activation of group-2 innate lymphoid cells. Proc Natl Acad Sci USA 111(43):15502–15507
Luzina IG, Pickering EM, Kopach P, Kang PH, Lockatell V, Todd NW et al (2012) Full-length IL-33 promotes inflammation but not Th2 response in vivo in an ST2-independent fashion. J Immunol (Baltimore, Md: 1950). 189(1):403–410
Wang Z, Ji N, Chen Z, Sun Z, Wu C, Yu W et al (2020) MiR-1165-3p suppresses Th2 differentiation via targeting IL-13 and PPM1A in a mouse model of allergic airway inflammation. Allergy, Asthma Immunol Res 12(5):859–876
Osterholzer JJ, Chen GH, Olszewski MA, Zhang YM, Curtis JL, Huffnagle GB et al (2011) Chemokine receptor 2-mediated accumulation of fungicidal exudate macrophages in mice that clear cryptococcal lung infection. Am J Pathol 178(1):198–211
Ubags NDJ, Suratt BT (2018) Isolation and characterization of mouse neutrophils. Methods Mol Biol (Clifton, NJ) 1809:45–57
Sun D, Zhang M, Liu G, Wu H, Zhu X, Zhou H et al (2016) Real-time imaging of interactions of neutrophils with Cryptococcus neoformans demonstrates a crucial role of complement C5a–C5aR signaling. Infect Immun 84(1):216–229
Leopold Wager CM, Hole CR, Wozniak KL, Wormley FL Jr (2016) Cryptococcus and phagocytes: complex interactions that influence disease outcome. Front Microbiol 7:105
Olszewski MA, Zhang Y, Huffnagle GB (2010) Mechanisms of cryptococcal virulence and persistence. Future Microbiol 5(8):1269–1288
Zheng CF, Ma LL, Jones GJ, Gill MJ, Krensky AM, Kubes P et al (2007) Cytotoxic CD4+ T cells use granulysin to kill Cryptococcus neoformans, and activation of this pathway is defective in HIV patients. Blood 109(5):2049–2057
Osterholzer JJ, Surana R, Milam JE, Montano GT, Chen GH, Sonstein J et al (2009) Cryptococcal urease promotes the accumulation of immature dendritic cells and a non-protective T2 immune response within the lung. Am J Pathol 174(3):932–943
Griesenauer B, Paczesny S (2017) The ST2/IL-33 axis in immune cells during inflammatory diseases. Front Immunol 8:475
Cayrol C, Girard JP (2018) Interleukin-33 (IL-33): a nuclear cytokine from the IL-1 family. Immunol Rev 281(1):154–168
Fanny M, Nascimento M, Baron L, Schricke C, Maillet I, Akbal M et al (2018) The IL-33 receptor ST2 regulates pulmonary inflammation and fibrosis to bleomycin. Front Immunol 9:1476
Nelson MP, Christmann BS, Werner JL, Metz AE, Trevor JL, Lowell CA et al (2011) IL-33 and M2a alveolar macrophages promote lung defense against the atypical fungal pathogen Pneumocystis murina. J Immunol (Baltimore, Md: 1950). 186(4):2372–2381
Sun D, Zhang M, Liu G, Wu H, Li C, Zhou H et al (2016) Intravascular clearance of disseminating Cryptococcus neoformans in the brain can be improved by enhancing neutrophil recruitment in mice. Eur J Immunol 46(7):1704–1714
Alves-Filho JC, Sônego F, Souto FO, Freitas A, Verri WA Jr, Auxiliadora-Martins M et al (2010) Interleukin-33 attenuates sepsis by enhancing neutrophil influx to the site of infection. Nat Med 16(6):708–712
Lan F, Yuan B, Liu T, Luo X, Huang P, Liu Y et al (2016) Interleukin-33 facilitates neutrophil recruitment and bacterial clearance in S. aureus-caused peritonitis. Mol Immunol 72:74–80
Oguma T, Asano K, Tomomatsu K, Kodama M, Fukunaga K, Shiomi T et al (2011) Induction of mucin and MUC5AC expression by the protease activity of Aspergillus fumigatus in airway epithelial cells. J Immunol (Baltimore, Md: 1950). 187(2):999–1005
Southam DS, Ellis R, Wattie J, Glass W, Inman MD (2008) Goblet cell rebound and airway dysfunction with corticosteroid withdrawal in a mouse model of asthma. Am J Respir Crit Care Med 178(11):1115–1122
Nath P, Leung SY, Williams AS, Noble A, Xie S, McKenzie AN et al (2007) Complete inhibition of allergic airway inflammation and remodelling in quadruple IL-4/5/9/13-/- mice. Clin Exp Allergy 37(10):1427–1435
Saradna A, Do DC, Kumar S, Fu QL, Gao P (2018) Macrophage polarization and allergic asthma. Transl Res 191:1–14
Joshi AD, Oak SR, Hartigan AJ, Finn WG, Kunkel SL, Duffy KE et al (2010) Interleukin-33 contributes to both M1 and M2 chemokine marker expression in human macrophages. BMC Immunol 11:52
Biondo C, Midiri A, Messina L, Tomasello F, Garufi G, Catania MR et al (2005) MyD88 and TLR2, but not TLR4, are required for host defense against Cryptococcus neoformans. Eur J Immunol 35(3):870–878
Nishizaki T (2018) IL-33 suppresses GSK-3β activation through an ST2-independent MyD88/TRAF6/RIP/PI3K/Akt pathway. Heliyon 4(11):e00971
Yoshimoto T (2018) The hunt for the source of primary interleukin-4: how we discovered that natural killer T cells and basophils determine T helper type 2 cell differentiation in vivo. Front Immunol 9:716
Ikutani M, Yanagibashi T, Ogasawara M, Tsuneyama K, Yamamoto S, Hattori Y et al (2012) Identification of innate IL-5-producing cells and their role in lung eosinophil regulation and antitumor immunity. J Immunol 188(2):703–713
Wu CA, Peluso JJ, Zhu L, Lingenheld EG, Walker ST, Puddington L (2010) Bronchial epithelial cells produce IL-5: implications for local immune responses in the airways. Cell Immunol 264(1):32–41
Acknowledgements
This research was supported by the Precision Medicine Research of the National Key Research and Development Plan of China (2016YFC0905800), the National Natural Science Foundation of China (82171738, 81671563, 81770031, and 81970031), and the Jiangsu Provincial Health and Family Planning Commission Foundation (Q2017001).
Author information
Authors and Affiliations
Contributions
ZW performed animal experiments and drafted the paper. QM analyzed the data. JJ performed the in vitro experiments. XY, EZ, YT and HH helped with improving the methodology. MH contributed to the paper revision. NJ and MZ designed and supervised the project.
Corresponding authors
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare.
Additional information
Edited by Christian Bogdan.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
430_2022_755_MOESM1_ESM.tif
Supplementary file1 Figure S1 Flow cytometry for the gating strategy of cells in BALF. Flow cytometry shows the gating strategy of total cells (R2 gate) and neutrophils (R3 gate) of cryptococcal-infected mice in BALF. Refer to Figure 6B, 6C. (TIF 233 KB)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, Z., Ma, Q., Jiang, J. et al. A comparative study of IL-33 and its receptor ST2 in a C57BL/6 J mouse model of pulmonary Cryptococcus neoformans infection. Med Microbiol Immunol 212, 53–63 (2023). https://doi.org/10.1007/s00430-022-00755-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00430-022-00755-4