Is mastocytic colitis a specific clinical-pathological entity?
Accepted: 10 November 2022
HTML: 15
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Authors
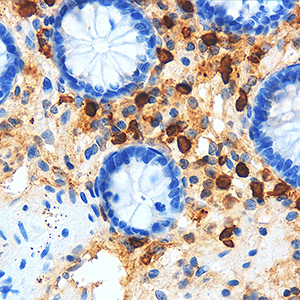
The number of intestinal mast cells (MC) is increased in several types of colitis, but the mucosa of patients with chronic non-bloody diarrhea has not been studied. The current study sought to determine the relationship between MC counts and degranulation and the severity of symptoms in patients with chronic loose stools. Following a negative laboratory workup for the most common causes of chronic diarrhea, patients with chronic non-bloody loose stools were included in the study. Patients with macroscopic evidence of inflammation or organic disease were excluded after endoscopy with biopsies. Biopsies from the 179 patients in the study were stained with hematoxylin and eosin and anti-CD117 c-kit antibodies. Immunohistochemistry was used to assess the degree of MC degranulation. Out of the 179 patients, 128 had normal histologic findings suggestive of irritable bowel syndrome and were used as controls. Twenty-four presented with abnormally high MC counts (≥40 MC x HPF), 23 with ≥20 intraepithelial lymphocytes x HPF suggesting lymphocytic colitis, and 4 had both (≥40 MC and ≥20 intraepithelial lymphocytes x HPF). In the patients with high MC counts, figures were significantly higher in the right colon versus the left colon (p=0.016), but degranulation did not differ in the right versus the left colon (p=0.125). No age or sex-related difference was observed (p=0.527 and p=0.859 respectively). The prevalence of abdominal pain and bloating did not differ in the three groups (p=0.959 and p=0.140, respectively). Patients with lymphocytic colitis (p=0.008) and those with high MC counts (p=0.025) had significantly higher evacuation rates compared to controls. There was no difference between these two groups (p=0.831). Mast cell degranulation was not associated with the number of evacuations, abdominal pain, or bloating (p=0.51; p=0.41; p=0.42, respectively). The finding that a significantly higher number of evacuations was linked to increased MC in the colonic mucosa of a subset of patients with otherwise normal laboratory and endoscopic findings suggests that "mastocytic colitis" may be a new clinical-pathological entity responsible for chronic non-bloody diarrhea. Prospective studies with a larger number of patients, as well as endoscopic and histological follow-up, are needed to confirm this hypothesis.
Ethics Approval
The study was performed with the institutional review board’s approval (Prot. N. 49353/2018).How to Cite

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
PAGEPress has chosen to apply the Creative Commons Attribution NonCommercial 4.0 International License (CC BY-NC 4.0) to all manuscripts to be published.

 https://doi.org/10.4081/ejh.2022.3499
https://doi.org/10.4081/ejh.2022.3499






